Based on Your Reading:
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

Tumor Treating Fields, also known as TTFields, is a new cancer therapy approved by the U.S. Food and Drug Administration to treat pleural mesothelioma. It works in combination with chemotherapy to limit cancer growth and improve survival.
Written by Karen Selby, RN • Edited By Walter Pacheco • Medically Reviewed By Dr. Andrea Wolf
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, February 2). Tumor Treating Fields (TTFields) and Mesothelioma. Asbestos.com. Retrieved April 19, 2024, from https://www.asbestos.com/treatment/ttfields/
Selby, Karen. "Tumor Treating Fields (TTFields) and Mesothelioma." Asbestos.com, 2 Feb 2024, https://www.asbestos.com/treatment/ttfields/.
Selby, Karen. "Tumor Treating Fields (TTFields) and Mesothelioma." Asbestos.com. Last modified February 2, 2024. https://www.asbestos.com/treatment/ttfields/.
Tumor Treating Fields is a new type of anti-cancer therapy that uses alternating electrical fields to limit cancer growth. It is combined with chemotherapy to control the growth of pleural mesothelioma.
In May 2019, TTFields became the second pleural mesothelioma treatment to be approved by the U.S. Food and Drug Administration.
The first mesothelioma treatment approved by the FDA was a chemotherapy regimen combining Alimta (pemetrexed) with cisplatin. It was approved in 2004. TTFields is administered in combination with this chemotherapy regimen.
A company called Novocure invented the noninvasive technology. It has been developing Tumor Treating Fields since 2000. TTFields received its first approval from the FDA in 2011 to treat a type of brain cancer called glioblastoma.
The clinical trial that led the FDA to approve TTFields for pleural mesothelioma reported an overall survival of 18.2 months.
Patients in the trial who received only chemotherapy lived 12.1 months. The addition of TTFields helped patients live an average of six months longer.
TTFields doesn’t offer a cure for mesothelioma, but it can significantly improve survival with a low risk of side effects.
Tumor Treating Fields is a novel therapy different from chemotherapy or radiation therapy. Basically, low-voltage electrical fields are safely applied through the skin to disrupt the microelectrical currents inside cells required for their division. It ‘jams up’ those fields inside tumor cells, not allowing them to divide, multiply or grow.Dr. Jacques FontaineThoracic Surgeon for Mesothelioma
Tumor Treating Fields applies alternating electrical fields of an intermediate frequency that are low in intensity.
The electrical fields are delivered through insulated pads that adhere to skin. The electrical field frequency is 100 to 300 kilohertz, and the intensity is delivered at 1 to 3 volts per centimeter.
Research shows that applying this range of alternating frequencies to cancer cells has the power to disrupt certain proteins that are essential to cell division.
When the electrical fields enter a cancer cell, they prevent these proteins from functioning properly. This stops cancer cells from multiplying, which limits tumor growth and may kill some cancer cells.
A a 2021 study published in Lung Cancer helps further explain why TTFields improves chemotherapy results. Researchers disovered the addition of TTFields to chemotherapy increases the expression of proteins that damage cancer DNA and reduces the level of proteins that repair cancer DNA. Damaged cancer DNA leads to tumor cell death.
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

Novocure’s TTFields delivery system for mesothelioma, branded under the name Optune Lua (previously known as the NovoTTF-100L System), is a portable device that can be used anywhere.
The system comes with a TTFields-generating device, battery charger and rechargeable batteries, power supply, insulated pads and a carrying bag.
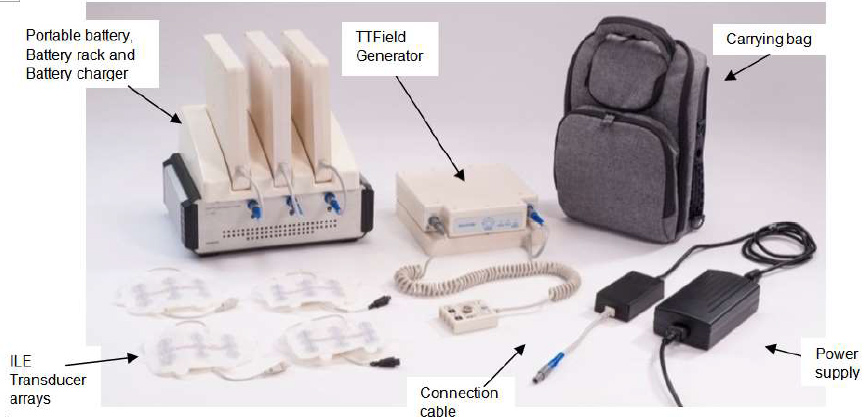
The treatment is designed for continual use at home or on-the-go. The carrying bag can be worn as a backpack or held in your hand.
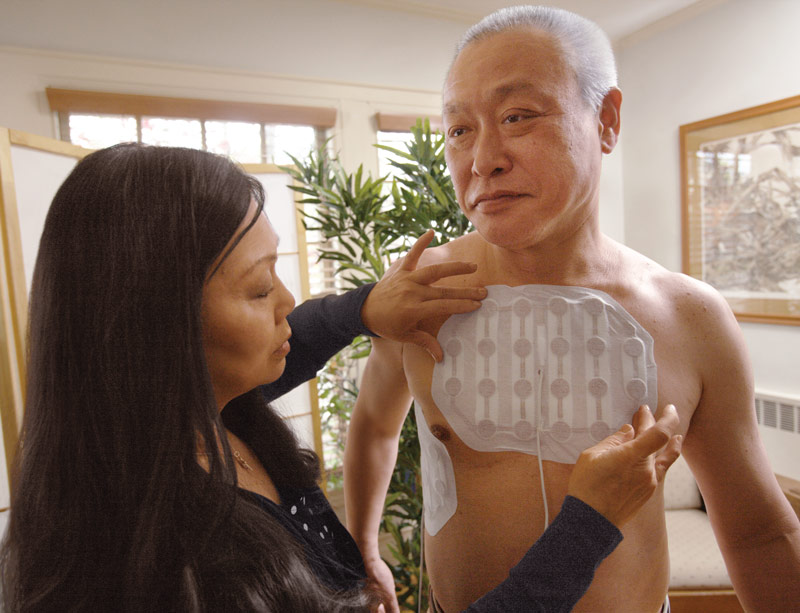
The therapy is applied with four pads attached to the front and back of a patient’s chest. The pads are changed every two to three days, and the area must be shaved. It involves no skin incisions or insertion of any medical devices into the body.
Patients are advised to wear NovoTTF-100L for 18 to 20 hours a day until their cancer progresses.
Patients can carry on with their lives while receiving treatment. They can run errands, take walks and do house chores while the therapy is administered.
However, keeping the device charged may present some logistical challenges for active people. NovoTTF-100L is plugged into a power supply or uses a large rechargeable battery pack that must be carried to power the device.
Every three weeks, patients will receive chemotherapy with Alimta and cisplatin or carboplatin for six cycles. Research shows that using NovoTTF-100L does not increase the toxicity of chemotherapy.
NovoTTF-100L is only prescribed with chemotherapy. That means patients must take chemotherapy to use the device.
The most common side effects of TTFields plus chemotherapy in pleural mesothelioma patients include:
Less common side effects include:
Skin irritation is reported in nearly half of patients who receive the therapy, but only 4% of patients report a severe level of irritation.
Do not use TTFields if:
The NovoTTF-100L System can only be prescribed by a mesothelioma doctor that has completed required certification training.
Patients with an underlying serious skin condition on the chest may not be able to receive Novocure’s mesothelioma treatment.
The clinical trial that led the FDA to approve NovoTTF-100L for pleural mesothelioma reported the following:
In October 2019, a patient at West Cancer Center in Memphis, Tennessee, became the first pleural mesothelioma patient outside of a clinical trial to use NovoTTF-100L since it was approved by the FDA.
Since February 2020, over 40 cancer centers across the U.S. have become certified to prescribe TTFields to patients with pleural mesothelioma. Many more centers are also seeking approval to offer the device.
“We are always looking for treatments to improve the survival, quality of life and options for patients,” said thoracic surgeon Dr. Taylor Ripley, director of the Mesothelioma Treatment Center at Baylor College of Medicine. “This provides another therapeutic option that may be valuable.”
Treatment Centers Certified for Tumor Treating Fields
| Treatment Center | doctors |
|---|---|
| Allegheny General Hospital | Dr. Zachary Horne Dr. Tom Colonias |
| Baylor College of Medicine | Dr. Pavan Jhaveri |
| Beaumont Hospital | Dr. Craig Stevens Dr. Zachary Seymour |
| Cleveland Clinic | Dr. Gregory Videtic |
| Frye Regional Medical Center | Dr. John O. DelCharco |
| Loyola University Medical Center | Dr. Tamer Abdelrhman Dr. Raymond Wynn |
| Miami Cancer Institute | Dr. Rupesh Kotecha |
| Ochsner Medical Center | Dr. Rockne Hymel Dr. Clayton Smith |
| Oregon Health and Science University | Dr. John Holland Dr. Josh Walker |
| Sylvester Comprehensive Cancer Center | Dr. Alan Dal Pra Dr. Derek Isrow |
| West Cancer Clinic | Dr. Matthew Ballo Dr. Cilla Edmonston Dr. Wes Garner Dr. Yuefeng Wang |
Novocure’s other TTFields device, called Optune, is specially designed to treat glioblastoma. It cannot be used to treat mesothelioma.
If you are interested in TTFields, ask your doctor if they are certified to prescribe the NovoTTF-100L System, and ask if you qualify for the therapy. The FDA has approved it only for pleural mesothelioma patients with inoperable tumors.
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
