Based on Your Reading:
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

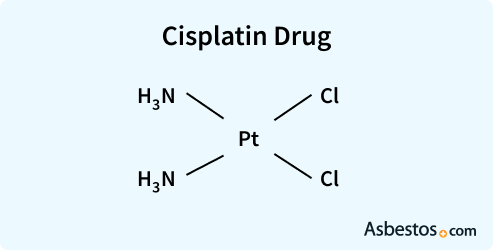
Cisplatin is a chemotherapy drug used to treat mesothelioma. It is commonly used alongside other chemo drugs to shrink tumors and kill cancer cells.
Written by Karen Selby, RN • Edited By Walter Pacheco • Medically Reviewed By Dr. Arkadiusz Z. Dudek
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, February 2). Cisplatin. Asbestos.com. Retrieved April 19, 2024, from https://www.asbestos.com/treatment/chemotherapy/cisplatin/
Selby, Karen. "Cisplatin." Asbestos.com, 2 Feb 2024, https://www.asbestos.com/treatment/chemotherapy/cisplatin/.
Selby, Karen. "Cisplatin." Asbestos.com. Last modified February 2, 2024. https://www.asbestos.com/treatment/chemotherapy/cisplatin/.
Cisplatin is one of the most widely used chemotherapy drugs for treating mesothelioma. When combined with pemetrexed (Alimta), it leads to the longest survival rates of any chemotherapy combination tested on mesothelioma.
Cisplatin was approved by the U.S. Food and Drug Administration in 1978 to treat testicular cancer and is still used in the treatment of several cancers today.
When it is given alone, cisplatin has a relatively low response rate of less than 15% in most people with pleural mesothelioma. However, when combined with other chemotherapy medications, patients experience improved response rates.
For example, the combination of cisplatin and pemetrexed (Alimta) is the most effective chemotherapy regimen for pleural mesothelioma. Treatment with this therapy has been shown to prolong disease control and survival.
| Name | Platinol |
| Alternate Names | Cisplatinum, Platamin, Neoplatin, Cismaplat |
| Manufacturer | Bristol-Myers Squibb |
| Dosage | 75 mg/m² every three weeks |
| Administration Route | Intravenous |
| Active Ingredient | Cisplatin |
| Drug Class | Antineoplastic alkylating agent |
| Medical Code | J9060 |
| Related Drug | Carboplatin |
| Interacting Drug | Anti-seizure medications, aminoglycoside antibiotics, amphotericin B, certain diuretics, nalidixic acid, pyridoxine, altretamine |
| Medical Studies | Study of Cytoreductive Surgery and Hyperthermic Intraoperative Chemotherapy with Pemetrexed and Cisplatin for Malignant Pleural Mesothelioma |
| FDA Warning | Renal toxicity, myelosuppression, nausea, vomiting, peripheral neuropathy, ear toxicity, allergic reactions, fetal harm |
Throughout treatment, mesothelioma patients receive the combination of cisplatin and pemetrexed every 21 days. The medication pemetrexed is given through an IV and typically takes about 10 to 15 minutes to complete. A dose of cisplatin delivered intravenously follows this step and usually takes about two hours to administer. The dose and number of cycles required will depend on the patient’s response to treatment as well as any side effects experienced.
The basic use of cisplatin is to promote apoptosis, also known as cell death.
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

Many chemotherapy side effects will subside when treatment ends. If a side effect lingers long after treatment has ended, reach out to your oncologist and explain your condition so they may prescribe medication to help you recover.
Side effects that may be experienced by mesothelioma patients receiving the chemotherapy drug cisplatin include:
Mesothelioma patients who experience any of the following symptoms after receiving cisplatin should notify their doctor:
In some cases, patients receiving this drug can experience extreme side effects such as severe organ damage. The central nervous system can also be affected by chemotherapy agents, leading to side effects such as “chemo brain,” headaches and psychological disturbances.
In unique cases, high doses of this drug combined with doxorubicin have been effective in shrinking mesothelioma tumors in half. Three small trials using more conventional doses of the two drugs produced positive response rates, but the results await confirmation from larger mesothelioma clinical trials.
A 2016 phase III clinical trial added bevacizumab to cisplatin and pemetrexed with a positive impact on overall survival. Pleural mesothelioma patients who received all three drugs lived an average of 18.8 months compared to 16.1 months for those who only received cisplatin and pemetrexed.
In 1999, Dr. David Sugarbaker reported a five-year survival rate of 46% in patients with epithelial cell type, lymph nodes free of cancer and clean surgical margins (the latter meaning that little to no cancer cells were detected post-surgery). Most of the participants in this study received cisplatin in combination with doxorubicin and cyclophosphamide after surgery, with radiation therapy following chemotherapy.
In a 1996 study, Sugarbaker and other researchers combined extrapleural pneumonectomy with radiation therapy and chemotherapy with cisplatin, doxorubicin and cyclophosphamide in 120 patients. The overall survival was 45% at two years and 22% at five years.
The combination of cisplatin and gemcitabine was studied after initial reports from Australia, where a partial response rate of 47% was observed. Median survival was 41 weeks. Most of the responses were seen in mesothelioma patients diagnosed with the epithelioid subtype, and symptom relief was correlated with response to treatment. When this regimen was compared to cisplatin and pemetrexed in Japanese patients, it generated only a 15% response rate and median overall survival of 306.5 days. In an Italian study, cisplatin and gemcitabine generated 26% response rates and median survival of 13 months.
Cisplatin and gemcitabine were also studied in combination with bevacizumab. In this triple-agent combination, partial response rate was similar to cisplatin and gemcitabine and placebo (24.5% for bevacizumab versus 21.8% for placebo), as was median overall survival (15.6 months for cisplatin, gemcitabine and bevacizumab versus 14.7 months for cisplatin, gemcitabine and placebo).
In a study of Tumor Treating Fields with pemetrexed and cisplatin or carboplatin, a median survival of 18.2 months was demonstrated.
Another trial studying the combination of cisplatin and pemetrexed with the immune checkpoint inhibitor durvalumab found that 57% of patients had no mesothelioma progression at six months.
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
