Based on Your Reading:
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

Clinical trials testing experimental and emerging treatments often are a mesothelioma patient’s best chance for extended survival. But enrolling in these research studies can be difficult. Limited funding and a lack of available trials present challenges for patients.
Written by Karen Selby, RN • Edited By Walter Pacheco • Medically Reviewed By Dr. Andrea Wolf
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, February 2). Importance, Obstacles & Stories of Mesothelioma Clinical Trials. Asbestos.com. Retrieved April 18, 2024, from https://www.asbestos.com/treatment/clinical-trials/participant-stories/
Selby, Karen. "Importance, Obstacles & Stories of Mesothelioma Clinical Trials." Asbestos.com, 2 Feb 2024, https://www.asbestos.com/treatment/clinical-trials/participant-stories/.
Selby, Karen. "Importance, Obstacles & Stories of Mesothelioma Clinical Trials." Asbestos.com. Last modified February 2, 2024. https://www.asbestos.com/treatment/clinical-trials/participant-stories/.
In July 2012, Richard M. faced a grim outlook on life. Doctors diagnosed him with mesothelioma. He had 12 months to live.
After undergoing an aggressive surgery, the Tennessee native turned to a targeted therapy clinical trial — and it worked. Now, he and his wife continue making the 100-mile trek to the ongoing clinical trial at the Sarah Cannon Research Institute in Nashville.
The 60-year-old praises that clinical trial for extending his life expectancy beyond his initial prognosis. He also knows his participation in the study provides hope for others seeking a cure for the rare, asbestos-related cancer.
People are the heart of these research studies. Without brave heroes like Richard, clinical trials wouldn’t be able to develop experimental and life-saving therapies that give other cancer patients longer lives and more precious time with their wives, husbands, daughters, sons and grandchildren.
Despite the path of hope clinical trials pave for thousands of people diagnosed with deadly mesothelioma, there are many road blocks along the way: Few mesothelioma clinical trials, lack of funding, long-distance travel to cancer centers, low patient and researcher participation, misconceptions and an overwhelming lack of awareness.
“With a clinical trial, you’re stepping into unknown territory,” Richard said. “But the choices I had were taking a known drug with a less-than-attractive success rate or an unknown drug to see what will happen.”
Richard chose the unknown.
While traditional treatments, such as radiation, chemotherapy and surgery, greatly improve the health and well-being of some mesothelioma patients, there are others who don’t respond well to these therapies or don’t qualify as candidates for a variety of reasons.
Mesothelioma survivor Kathy Angerman participated in a clinical trial in 2013 that stopped the growth of tumors for six months. She restarted chemotherapy in 2014. But after one year without tumors, she was forced to quit her treatment because the side effects wore her down.
“I’m not sure what I’m going to do if or when the doctor tells me the cancer is growing again,” Angerman said. “That’s why I wanted to find another clinical trial. Chemotherapy alone isn’t the answer.”
For patients who find themselves in similar situations as Angerman, clinical trials offer hope.
When doctors diagnosed 39-year-old Michelle with mesothelioma before Christmas, aggressive surgery was not an option because the cancer already had spread. Michelle asked that Asbestos.com not use her last name.
Michelle didn’t hesitate when her doctor suggested she participate in a clinical trial. She already knew the alternative.
“I just said, ‘let’s do it.’ After all, what other choice did I have?” Michelle said from her home near Toronto. “I could sit home and do nothing, but that wasn’t an option for me. You’ve got to fight this thing.”
In February, she enrolled in a clinical trial testing immunotherapy drug pembrolizumab, also known as Keytruda, at the Princess Margaret Cancer Centre in Toronto.
“I feel good right now, much better than I did when it started. I went up a flight of stairs and didn’t lose my breath like I had earlier,” Michelle said. “I felt proud of myself. It was like taking baby steps, but it was a big milestone for me.”
Dr. John Cho, radiation oncologist and mesothelioma specialist at Princess Margaret Cancer Centre, said clinical trials “are often where the newest treatments are going to be found.”
Each clinical trial has a distinct, well-focused purpose, often categorized into screening, prevention or treatment. They typically give patients access to the latest experimental drugs and novel treatments, helping increase survival rates.
Cancer patients should realize that a clinical trial investigating the potential benefits of an experimental treatment may be available only at tertiary specialty cancer centers. Also, these novel therapeutic trials may not be available at local cancer clinics or community hospitals.
Despite this specific limitation, there are other important benefits.
These studies give people a chance to play a more active role in their own health care. Many participants appreciate the opportunity to choose a new therapy from a list of clinical trials and possibly be among the first group of patients to benefit from these experimental treatments.
Clinical studies also may help participants feel safer during treatment. Mesothelioma trials usually take place at leading cancer centers and often are led by some of the top cancer specialists in the field.
Doctors with this level of expertise offer patients some clear advantages over general oncologists who may only see one or two cases of mesothelioma during their entire career.
In addition to a full team of specialists providing quality care to patients, research coordinators keep a watchful eye on side effects while ensuring the study follows all safety regulations.
Participants in clinical trials also tend to have more positive results than people who choose the standard treatment. Some key benefits include closer monitoring throughout treatment and easier access to advice and support from medical professionals.
Even when a clinical trial doesn’t provide effective treatment, some participants say they’re happy to be involved in research that will one day help another mesothelioma patient.
In 2014, Florida retiree Jack Riordan participated in a phase I dose-escalation trial involving an immunotherapy antibody. His body responded poorly to the drug, sending him into near convulsions and making him feel like he was freezing for the next 24 hours. Two weeks later, he did it again and had the same response.
Still, he did it a third time, knowing how bad it would make him feel, but understanding researchers would closely track his response.
“It was discouraging because his body just couldn’t handle it, but it still gave him hope that this would be helping someone else,” his wife Judy said. “That’s how great of a guy he was.”
Despite the bad experiences, he inquired about joining another trial, but his health worsened before he could enroll. Riordan died 15 months after his diagnosis. He was 75.
“For him, it was a last resort, but he wanted to keep trying,” Judy said. “Why not try and help others — even if it won’t help you. That’s how he thought.”
Get a Mesothelioma Treatment Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

As of January 2022, ClinicalTrials.gov, a website run by the U.S. National Institutes of Health (NIH), shows cancer centers across the nation are conducting 127 mesothelioma studies that are currently recruiting participants.
In the last few months, a number of these clinical trials have shown promise for mesothelioma patients, especially the study involving immunotherapy drug Keytruda.
This phase II trial at the University of Chicago Comprehensive Cancer Center tests the effectiveness of Keytruda, which stimulates the immune system to kill cancer cells. The drug is an antibody that blocks a protein called programmed cell death 1 (PD-1), which promotes an immune response against tumor cells. Keytruda put President Jimmy Carter’s melanoma into remission in 2015.
BAY94-9343 is a new kind of immunotherapy drug that combines an antibody with an anti-cancer compound. The antibody part of the drug helps deliver the anti-cancer portion directly to cancerous cells, known as targeted therapy. This type of drug causes few side effects because it targets cancer cells, not surrounding healthy cells. BAY94-9343 is one of the few anti-cancer drugs that impacts sarcomatoid and biphasic mesothelioma cell types. This multicenter clinical trial administers the drug intravenously once a week for three weeks in a row.
This multicenter trial tests the value of immunotherapy drug CRS-207, which targets mesothelin, a compound made by mesothelioma tumors. Researchers create CRS-207 by weakening and genetically modifying the bacterium Listeria monocytogenes so it doesn’t cause listeriosis, which infects the nervous system. Instead, it triggers a powerful immune response against cancer. Preliminary results show 94 percent of study participants responded positively to the drug with partial tumor shrinkage or no new tumor growth. Doctors administer CRS-207 prior to chemotherapy with pemetrexed and cisplatin.
This clinical trial examines the benefits of a drug that slows new tumor growth. Tumors spread by forming new blood vessels through which cancer cells can migrate, a process called angiogenesis. LY3023414 blocks angiogenesis. Mesothelioma patients participating in this multicenter trial also receive chemotherapy with pemetrexed and cisplatin.
Several clinical trials are now considered landmark studies that propelled the treatment of mesothelioma. Without these trials, doctors would still be searching for effective treatment options.
Results from these trials led to important advances in mesothelioma therapy. The pemetrexed and cisplatin trial revealed a promising survival advantage, convincing researchers to update the standard of care for mesothelioma chemotherapy.
The MARS trial led many surgeons to forego EPP for pleurectomy/decortication (P/D), a less aggressive, lung-sparing surgery. A follow-up MARS 2 trial is now underway in the U.K. to explore the benefits of P/D for mesothelioma.
The HIPEC trial revealed the procedure’s ability to remarkably improve life expectancy, with one peritoneal mesothelioma patient still alive 19 years later.
Despite the successes of previous clinical trials and the promising outlook of ongoing studies, researchers and patients enrolled in clinical trials face many challenges.
Whether it’s a lack of funding, low participation levels among patients and researchers or a misunderstanding of how clinical trials work, there is much that needs to be overcome to ensure innovations in research continue so current and future mesothelioma patients can live longer lives.
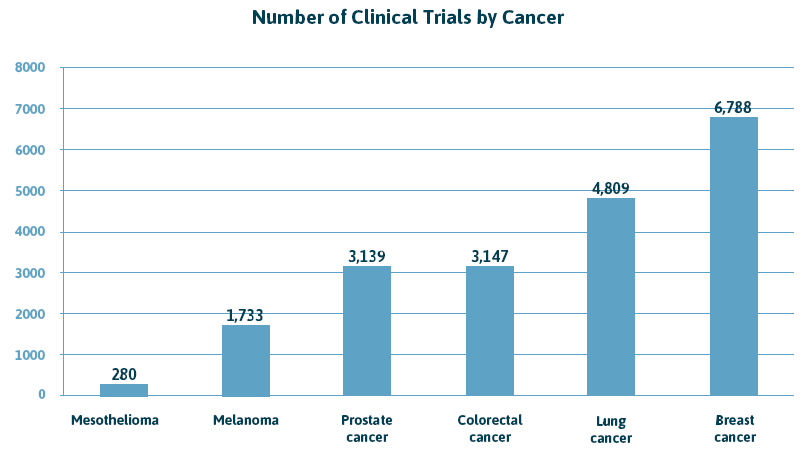
Breast, lung and prostate cancer generate plenty of publicity, research funding and clinical trials because hundreds of thousands of people are diagnosed every year with those cancers in the U.S.
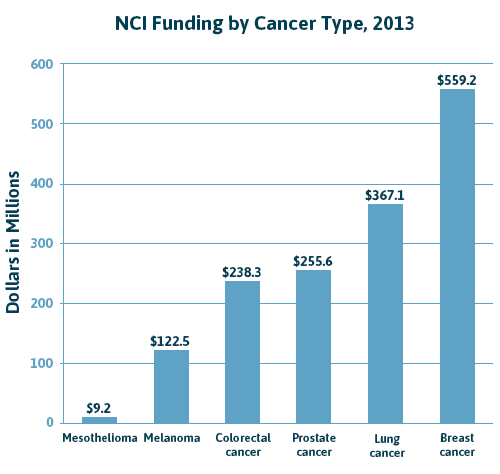
For example, the American Cancer Society estimates that 221,500 new lung cancer cases will be diagnosed in 2017. The National Cancer Institute (NCI) provided more than $331 million in 2017 to lung cancer researchers. There are also 1,659 clinical trials currently recruiting lung cancer patients, according to Clinicaltrials.gov.
Unfortunately, the rarity of mesothelioma means significantly fewer clinical trials and less funding.
Doctors diagnose about 3,000 new mesothelioma cases a year. The NCI provided $9 million in funding for research, and there are 98 clinical trials recruiting mesothelioma patients as of January 2018.
Although there is an abundance of outstanding mesothelioma research proposals, limited funds to support them has led to historically low rates of grant approvals.
From 1990 to present, the American Cancer Society received 30 mesothelioma research proposal applications. It approved grants for five of these for a total of more than $1.6 million dollars.
People have different beliefs and misconceptions about clinical trials that influence their decision to participate.
For example, people may fear that clinical trials actually benefit pharmaceutical companies more than cancer patients, or they suspect the medical system benefits more than participants.
These fears and misconceptions are completely normal and should be addressed with your oncologist or one of the researchers leading the clinical trial.
People may say they didn’t know joining a clinical trial was an option for them. This highlights how important it is to ask your treatment team about clinical trial options, especially if none of your doctors bring them up.
The idea of serving as a guinea pig for experimental and unproven treatments frightens some people with cancer. While clinical trials may involve uncomfortable tests and uncertainty about treatments to answer important medical questions, the vast majority of participants report positive experiences. Patients praise the high level of care they received, as well as close monitoring and round-the-clock access to medical professionals.
Michelle, who participated in the Canadian clinical trial in Toronto, debunks this myth.
“You could call me a guinea pig, but this cancer center doesn’t make you feel that way at all,” Michelle said. “They are constantly monitoring you. You can talk with someone 24/7 if you’ve got questions or concerns. You get great care. I’m a good patient for them. I’m young, healthy and have no other issues.”
Another common but unfounded fear is that people may join a trial only to receive a placebo or “sugar pill.” It should be noted the only time a placebo would ever be used as a randomized treatment in any type of clinical trial would be the extraordinarily rare circumstance when there are no recognized available therapeutic modalities for a given disease process.
This certainly does not apply mesothelioma in modern times. While a few clinical trials in the past used placebos in their studies, researchers still provided the best standard of cancer care to all participants.
For example, mesothelioma patient Peter L. participated in a clinical trial in New York City involving WT1 immunotherapy vaccine. Not only did researchers cancel the trial because of an overall lack of efficacy, but he later learned he was given a placebo and not the actual drug.
“I always thought I was getting the real thing,” Peter said. “It was good for me psychologically. But in reality, you just don’t know what you are getting in a double-blind trial. It’s Russian roulette.”
Peter felt disappointment. But he would consider another clinical trial if he thought it would help him or others in the future.
“I would not discourage people from going into a trial,” Peter said. “I still believe in the good they can do.”
Sometimes people don’t enroll in a clinical trial because they are afraid their health insurance won’t cover the costs. While coverage can vary depending on the company, state and federal laws require insurers to pay for some clinical trial treatment costs. Participants may still have to pay deductibles related to their standard-of-care treatment, so be sure to talk to your insurance company about what costs it will cover.
Too many people believe a clinical trial is only used when all other traditional methods have failed. The truth is clinical trials are available for people at any stage of mesothelioma. Some early-stage patients may benefit from clinical trials searching for innovative ways to diagnose and detect cancer. Clinical trials have a variety of focuses, even for patients in remission.
Some patients believe joining a clinical trial is for a short period of time. While some trials may end because of a lack of efficacy, many trials become longer treatment options if they are safe and effective. On the other hand, if the treatment is not effective or makes patients unhappy or unhealthy, they can drop out of treatment at any time.
Many precautions are taken before a clinical trial opens to ensure the safety of all participants. In addition to regular medical attention from trial staff and close observation, participants will continue to be monitored by their regular physicians. Throughout the study, researchers are required to notify patients of any new risks or side effects discovered.
Older patients are underrepresented in many clinical trials, but that is not because researchers don’t want them. Usually, it’s because many older patients are unable to travel far distances to participate. In fact, people over 60 are approximately 10 times more likely to be diagnosed with mesothelioma than people under 40. As a result, mesothelioma clinical trials typically enroll older patients.
With knowledge gained from clinical trials, researchers have developed effective cures for several cancers such as Hodgkin lymphoma and testicular cancer.
Clinical trials also drive innovation in the treatment of mesothelioma and other cancers, yet low participation rates hold back progress.
The lack of volunteers is the biggest barrier to completing clinical trials, according to the American Cancer Society. Fewer than 5 percent of adults with cancer volunteer for clinical trials, compared to 60 percent of children under age 15.
Higher participation from children helped researchers uncover a cure for childhood acute leukemia and significantly boosted survival rates for other childhood cancers during the past few decades.
To see the same results with adult cancers, doctors and researchers must educate patients about clinical trials and encourage them to join if they are good candidates.
Dr. Cary P. Gross, professor of medicine at Yale University and co-director of the Robert Wood Johnson Foundation Clinical Scholars, said low participation in mesothelioma clinical trials is a problem.
“For studies to produce meaningful results, you need a large number of people involved, which is why it’s so important for patients to participate if given the opportunity,” Gross said. “It’s critical for a rare cancer like mesothelioma.”
Every person with cancer has a unique situation, as well as their own reasons for wanting or not wanting to participate in a clinical trial. While it’s perfectly fine to forego clinical trials and pursue standard treatments, patients should ensure they are fully informed before making that decision.
Cancer centers in California, Illinois, Maryland, New York, Texas, Minnesota and Pennsylvania host the majority of clinical trials.
Mesothelioma patients living far from those states may face some challenges reaching these studies because of travel costs, or an inability to drive or travel by air.
Those living in these states may reside in cities too far from major metropolitan areas where these specialty cancer centers are usually located.
Angerman, who participated in a clinical trial at the University of Chicago Medical Center in 2013, lives three hours away from the center. Luckily, her son drove her to the clinical trial twice a month. But he has since moved out of state for a new job, and it couldn’t have come at a worse time for his mother.
The Chicago medical center is now recruiting mesothelioma patients for a new clinical trial that may keep Angerman’s aggressive cancer at bay. But without her son or anyone else to drive her this time, the 72-year-old admits the distance is too great for her to navigate on her own.
Clinical trials are going to be the avenue to making progress with this disease. I think they are great. My only complaint is that they are not available closer to home. I just can’t get there.Kathy AngermanMesothelioma Survivor
Because of the fast-moving nature of mesothelioma, Angerman is running out of time without access to the clinical trial.
“For me, I don’t think that trial is an option,” she said. “I really don’t know what I’m going to do.”
Unfortunately, there is a significant lack of awareness about new clinical trials.
There are patients who don’t know trials are available, and doctors who may be unaware of mesothelioma trials near them. The uncertainty leads to low involvement in clinical trials and less of a fighting chance to find a cure.
For many general practitioners, their only mesothelioma exposure is a quick overview in medical school. Because of the rarity of the cancer and the difficult diagnosis process, many doctors may be slow to identify mesothelioma, let alone spend the time researching new treatment options available.
“Many physicians simply are unaware of clinical trials that might benefit their patient,” said Dr. Judy Stone, an infectious disease doctor who as both led and participated in clinical trials. “Further, with the increasing pressure to see patients more and more quickly, they simply don’t have the time to engage in lengthy discussions with patients.”
Because of the great divide between mesothelioma research and primary care physicians, patients with the aggressive cancer may not have a chance to join a clinical trial that may extend their lives.
The solution: Increased mesothelioma awareness must be a priority among physicians, and mesothelioma patients need to seek a mesothelioma specialist for treatment.
Finding a mesothelioma specialist can make a huge difference in a patient’s diagnosis, prognosis and treatment options, including clinical trials. Specialists will know of any possible clinical trials that a patient may qualify for.
“Awareness is a big problem sometimes,” Cho said. “Even if a treatment is available through a clinical trial, if you can’t access it, it’s almost like it wasn’t even available in the first place.”


Deciding whether a clinical trial is the right choice for a mesothelioma patient is an important decision to make, and it’s not to be taken lightly.
A patient’s health care team can help explain the process and answer any questions. There are also some important questions patients should ask themselves before making a decision. Some may be difficult to answer, but it helps to start thinking about them.
Joining a clinical trial might be the most important step a mesothelioma patient can take. It often is a leap of faith that goes well beyond one’s own best interest.
It’s what makes progress possible. It’s what fuels the engine of hope. It’s what creates the optimism for the future.
Dr. Claire Verschraegen at the University of Vermont Medical Center says one of the first things patients tell her after she suggests clinical trials is, “You want me to be a guinea pig?”
“And I say, ‘No, no, no. I want you to be a hero,’” Verschraegen said. “A hero is someone who puts his own life out there to help others, someone who really gives of himself. They become heroes for trying, because even if it doesn’t help them, it helps those that follow.”
Mesothelioma patient Michelle is one of these heroes.
“Maybe we can do something about it, or at least help others in the process,” she said. “Maybe we can find something to put this thing to sleep. That’s what these trials are about.”
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
