FDA Approves First New Treatment for Mesothelioma in 15 Years
Treatment & DoctorsWritten by Matt Mauney | Edited By Walter Pacheco

The U.S. Food & Drug Administration on Thursday approved Tumor Treating Fields, a therapy involving electric currents that disrupt cancer cell division and inhibit tumor growth, for the first-line treatment of malignant pleural mesothelioma.
It is the first new treatment for mesothelioma in more than 15 years that the FDA has approved. In 2004, the agency added the chemotherapy drug pemetrexed (Alimta) to standard-of-care treatment.
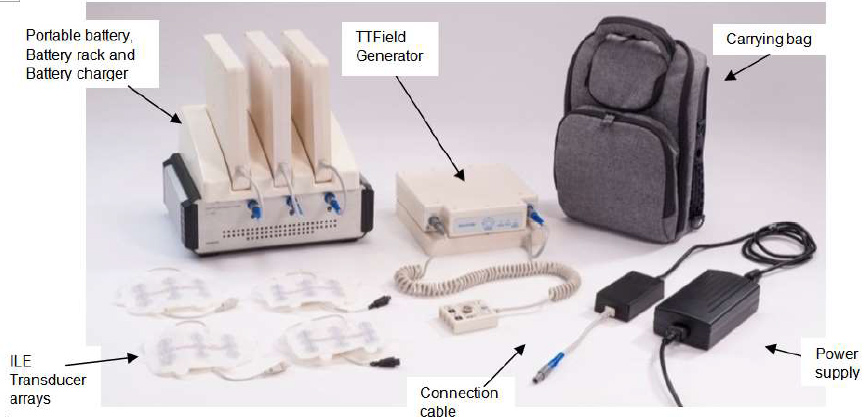
The FDA approved Novocure’s NovoTTF-100L System under the agency’s Humanitarian Device Exemption. The approval comes eight months after Novocure, which manufactures and markets the device, released results from its STELLAR phase II clinical trial.
The Tumor Treating Fields delivery system is now FDA approved in combination with pemetrexed plus platinum-based chemotherapy for the first-line treatment of unresectable, locally advanced or metastatic malignant pleural mesothelioma (MPM).
It is a major breakthrough for the rare cancer caused by asbestos exposure. Only 10% to 20% of mesothelioma patients qualify for tumor-removing surgery.
“Since 2000, we have been developing and commercializing Tumor Treating Fields to extend survivals in some of the most aggressive forms of cancer,” Bill Doyle, Novocure’s executive chairman, said in a press release. “FDA approval of NovoTTF-100L provides patients with the first FDA-approved treatment for MPM in more than 15 years and, as our first FDA-approved torso cancer indication, marks a major milestone for Novocure.”
Access top mesothelioma cancer centers that have experience treating this rare disease.
Get Help NowHow NovoTTF-100L Works
Tumor Treating Fields is a relatively new technology that has gained traction as a treatment for some of the most aggressive cancers.
NovoTTF-100L uses low-intensity alternating electric fields, which are tuned to interfere with the division of cancer cells. For mesothelioma, the currents are delivered noninvasively to the upper torso.
The system is intended for continuous home use by mesothelioma patients.
In 2011, the FDA approved Optune, another Tumor Treating Fields device, under the Premarket Authorization pathway for the treatment of glioblastoma, the most aggressive form of brain cancer.
“Our mission is to improve patients’ survival without the toxicity you get with systemic chemotherapy,” Dr. Eilon Kirson, chief science officer at Novocure, told The Mesothelioma Center at Asbestos.com in September 2018.
Approval Comes After STELLAR Results
Novocure’s STELLAR clinical trial saw significant survival benefits for pleural mesothelioma patients who used Tumor Treating Fields in combination with standard chemotherapy.
Patients treated with the system survived six months longer than patients receiving only chemotherapy. There were no reported cases of major side effects or system toxicity.
- Tumor Treating Fields Plus Chemo: 18.2-month overall survival
- Alimta and Either Cisplatin or Carboplatin Only: 12.1-month overall survival
More than 97% of patients saw a clinical benefit, which includes either partial response or stable disease.
The median overall survival for patients with epithelioid mesothelioma — the most common mesothelioma cell type — was 21.2 months. Patients with sarcomatoid or biphasic cell types survived an average of 12.1 months.
Patients in the Tumor Treating Fields group saw a progression-free survival of 7.6 months, compared to 5.7 months for the chemotherapy only group.
Skin irritation was the most reported side effect from the NovoTTF-100L system, seen in 46% of patients. But only 4% reported grade 3 skin irritation.
The clinical trial, held at cancer centers across Europe, included 80 patients with unresectable, previously untreated, malignant pleural mesothelioma. The results were presented in September 2018 at the 19th annual World Conference on Lung Cancer.
“It’s not the cure we’re all looking for, but it means that some patients are seeing a significant benefit,” Kirson said. “It’s a jump forward. It can change the outcome of this disease, make it that much less horrific.”