Tumor Treating Fields (TTFields) and Mesothelioma
Tumor Treating Fields, also known as TTFields, is a newer cancer therapy approved by the U.S. Food and Drug Administration to treat pleural mesothelioma. It works in combination with chemotherapy to limit cancer growth and improve survival.
What Are Tumor Treating Fields for Mesothelioma?
Tumor Treating Fields for mesothelioma is a noninvasive therapy that uses electrical fields to target cancer cells. Electrical fields come from tiny charged particles like electrons in your body. These fields can push or pull on other charged particles similar to how magnets can attract or repel each other.
In mesothelioma treatment, TTFields use these electrical fields to target cancer cells without harming your healthy cells. They disrupt special proteins inside mesothelioma cells that help them divide and grow. When the fields interfere with this process, mesothelioma cells eventually die, which can help shrink your tumors over time.
Key Facts About TTFields for Mesothelioma
- TTFields help chemotherapy work better. It makes mesothelioma cell walls easier to pass through, so chemo can get in and kill the cancer cells more effectively.
- TTFields might help stop mesothelioma tumors from regrowing after treatment.
- A small, portable device sends mild electrical fields through the skin, and patients can go about their day while using it.
The U.S. Food and Drug Administration approved TTFields for pleural mesothelioma in May 2019. TTFields therapy is given along with a chemotherapy regimen of Alimta (pemetrexed) and cisplatin. Novocure has been developing TTFields since 2000.
The clinical trial that led to FDA approval reported overall survival for people with pleural mesothelioma was an average of 6 months more with TTFields than with chemo alone. TTFields therapy doesn’t offer a cure for mesothelioma, but it can extend survival with a low risk of side effects.
How Does TTFields Treat Mesothelioma?
TTFields therapy sends gentle electrical waves through the skin to help treat mesothelioma. The electrical waves switch directions back and forth repeatedly, known as alternating electrical fields.
A 2025 study in The American Journal of Cancer Research supported previous findings that TTFields boosts chemo results. The study found TTFields therapy helps cancer cells take in more medicine and keep it inside longer.
A 2021 study in Lung Cancer also found adding TTFields to chemo increases the amount of proteins that damage cancer cells’ DNA and reduces the level of proteins that repair cancer DNA. Damaged cancer DNA leads to tumor cell death.
As Dr. Jacques Fontaine, director of Moffitt Cancer Center’s Mesothelioma Research and Treatment Center, tells us, “Basically, low-voltage electrical fields are safely applied through the skin to disrupt the electric microcurrents inside cells required for their division. It ‘jams up’ those fields inside tumor cells, not allowing them to divide, multiply or grow.”
How Is TTFields Administered?
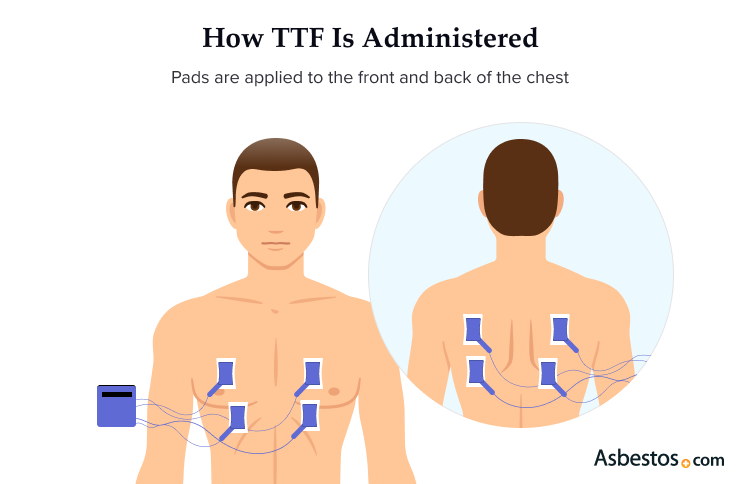
TTFields delivers electrical waves through insulated pads that stick to your skin. After your mesothelioma specialist prescribes TTFields therapy for you, a Novocure device support specialist will arrange for in-person training for you and your caregiver. Novocure’s TTFields delivery system is branded under Optune Lua and is a transportable device that can be used anywhere.
The system includes a TTFields-generating device, battery charger and rechargeable batteries, power supply, insulated pads and a carrying bag. The frequency of the electrical field is 100 to 300 kilohertz, and the intensity is delivered at 1 to 3 volts per centimeter.
Your TTFields mesothelioma treatment is designed for continual use for 18 to 20 hours a day at home or on the go. You can run errands, take walks or do household chores while it’s administered. The carrying bag can be worn as a backpack or held in your hand. There are 4 pads attached to the front and back of your chest and back. The pads are changed every 2 to 3 days, and the area must be shaved. It involves no skin incisions or inserting any medical devices into the body.
Keeping the device charged can present some logistical challenges for active people. The delivery system is plugged into a power supply or uses a large rechargeable battery pack that must be carried to power the device. Every 3 weeks, people with mesothelioma will receive TTFields therapy with Alimta and cisplatin or carboplatin for 6 cycles. Research shows TTFields doesn’t increase the toxicity of chemo.
Side Effects of TTFields for Mesothelioma
Skin redness and irritation are the most common side effects of TTFields therapy for mesothelioma. People may also experience side effects related to the chemotherapy they receive while prescribed TTFields.
Possible Side Effects of TTFields
- Allergic reaction to the adhesive patches
- Anemia
- Chest pain
- Constipation
- Cough
- Fatigue
- Itchy skin
- Muscle twitching
- Nausea
- Pain, burns or ulcers
- Skin infection or toxicity
- Tingling sensation under the patches
Nearly 50% of patients who receive the therapy report skin irritation. Only 4% report a severe level of irritation. Discuss any side effects with your health care team if you notice new or worsening issues.
Managing Side Effects of TTFields for Mesothelioma
If you experience side effects from your mesothelioma treatment with TTFields, ask your doctor to recommend ways to manage them. Approaches to managing these effects are usually topical.
Avoiding and Managing TTFields Side Effects
- Pad replacement: Replace the pads as recommended to maintain skin health.
- Proper skin care: Clean and dry your skin thoroughly before applying the pads to reduce irritation.
- Rotate placement: Regularly change the position of the pads to avoid prolonged pressure on one area.
- Topical steroids: For moderate irritation, your doctor may prescribe topical corticosteroids like hydrocortisone.
- Use barrier creams: Apply skin-protective creams to reduce friction and irritation.
It’s important to monitor your skin for signs of infection. These signs can include warmth, swelling or pus. Seek medical attention if you experience these effects.

Connect with trusted specialists who truly care about your health. Get fast, stress-free appointment help.
Find a Doctor NowWho Is Eligible for TTFields Treatment for Mesothelioma?
TTFields therapy for pleural mesothelioma is best for people in good overall health who can wear the device for 18 to 20 hours per day. People free of skin conditions such as infections, severe dermatitis or chest burns are more likely to be eligible for TTFields therapy.
People with an active implanted medical device, such as a pacemaker, may not be eligible. TTFields can interfere with the function of these medical devices. Pregnant women or those trying to conceive shouldn’t use TTFields. Its effects on fetal development aren’t well known.
For safety, only a mesothelioma doctor who has completed the required certification training can prescribe TTFields. Novocure’s training covers device operation, patient selection, potential side effects and troubleshooting.
Effectiveness of TTFields for Mesothelioma
A pivotal clinical study, the STELLAR trial, showed the benefits of TTFields Benefits Shown in STELLAR Clinical Trial.
- Median overall survival with TTFields and chemo was 18.2 months vs. 12.1 months with chemo alone.
- The 1-year survival rate was 62% with TTFields plus chemo vs. approximately 50% for chemo alone.
- Participants had no new cancer growth for an average of 7.6 months with TTFields vs. 5.7 months with chemo alone.
- More than 97% of people responded to TTFT with tumor shrinkage or no new tumor growth.
- Patients with epithelial mesothelioma, which responds best to treatment, lived 21.2 months.
Scientists are also exploring the possible benefits of using TTFields with therapies like immunotherapy to see if these combos can work even better. As research continues, TTFields may become an essential option for mesothelioma care, offering new hope to patients.
How to Receive TTFields Treatment for Mesothelioma
As of August 2024, TTFields therapy is available at more than 500 select cancer treatment centers. Many more centers are also seeking approval to offer the device. If you’re interested in TTFields, ask your doctor if they’re certified to prescribe it and if you’re eligible.
“We are always looking for treatments to improve the survival, quality of life and options for patients,” said thoracic surgeon Dr. Taylor Ripley, director of the Mesothelioma Treatment Center at Baylor College of Medicine. “This provides another therapeutic option that may be valuable.”
In 2019, the first person with pleural mesothelioma was able to use Novocure’s Optune Lua delivery system outside of a clinical trial. The person was a patient at West Cancer Center in Memphis, Tennessee.
Common Questions About TTFields for Mesothelioma
- How much does TTFields cost?
-
The cost of TTFields therapy can be significant, often ranging from $10,000 to $20,000 per month, depending on the region and healthcare system. The transportable delivery system, which delivers TTFields for mesothelioma patients, is a specialized medical device and its cost includes the device, contact pads and ongoing support.
- Does insurance cover TTFields?
-
Coverage may vary depending on the insurance provider and the patient’s specific plan. Prior authorization is required. Patient Advocates, your health care team and patient support from the manufacturer, Novocure, can help you navigate insurance coverage and financial assistance programs.
- How long do mesothelioma patients use TTFields?
-
People with mesothelioma usually use TTFields continuously for 18 to 20 hours per day throughout their treatment course. The duration varies depending on your response to treatment and overall treatment plan.
In clinical studies, patients often used TTFields for 12 months or longer. Treatment continues as long as it’s tolerated and you benefit from it.
- Can TTFields be used without chemotherapy?
-
TTFields are most effective for treating pleural mesothelioma when used in combination with chemo, particularly Alimta (pemetrexed) and cisplatin.
A mesothelioma specialist’s decision to use TTFields without chemo would depend on your specific case and treatment goals.





