Immune Checkpoint Inhibitors for Mesothelioma
Immune checkpoint inhibitors are a type of immunotherapy for mesothelioma. Cancer uses the body’s “off switches” to hide. These drugs block those switches, helping the immune system find and attack the cancer. They give people more options and hope.
What Are Immune Checkpoint Inhibitors?
Immune checkpoint inhibitors are a type of immunotherapy that treats pleural mesothelioma. They help your immune system find and fight this aggressive cancer. Mesothelioma cells use a special signal (PD-L1) to connect to a protein (PD-1). This protein works like an off switch on T cells, a key part of your body’s defense system.
Blocking these signals, immune checkpoint inhibitors help T cells stay active. Immunotherapies like Opdivo (nivolumab) and Keytruda (pembrolizumab) block PD-1. Similarly, Yervoy (ipilimumab) and Imjudo (tremelimumab) block CTLA-4. This protein turns off T cells earlier in the body’s immune response.
Types of Checkpoint Inhibitors for Mesothelioma
Experts classify checkpoint inhibitors into groups based on their targets. For example, PD-1 inhibitors stop the PD-1 signal. This helps T cells find and attack mesothelioma cells.
Checkpoint Inhibitors for Mesothelioma
- Keytruda (pembrolizumab) is a PD-1 inhibitor.
- Opdivo (nivolumab) is a PD-1 inhibitor.
- Yervoy (ipilimumab) is a CTLA-4 inhibitor.
The most common drugs are Keytruda, Opdivo and Yervoy, which are all Food and Drug Administration-approved for first-line or second-line unresectable pleural mesothelioma. These drugs are also monoclonal antibodies. They’re synthetic but work like the body’s natural antibodies, which help fight disease.
Other checkpoint inhibitors are being studied. These include: Bavencio (avelumab), Imfinzi (durvalumab), Imjudo (tremelimumab) and Tecentriq (atezolizumab). All are PD-1 inhibitors, except for Imjudo, which is a CTLA-4 inhibitor.
How Do Checkpoint Inhibitors Work for Mesothelioma?
One of the most common signals mesothelioma takes over is the PD-1/PD-L1 pathway. Checkpoint inhibitors block mesothelioma tumors from connecting with these proteins to “turn off” the immune system. These drugs turn T cells back on.
When T cells are turned back on, they can kill mesothelioma cells. This type of treatment helps the immune system fight the cancer. It’s a good option when the disease is advanced and surgery can’t be done.
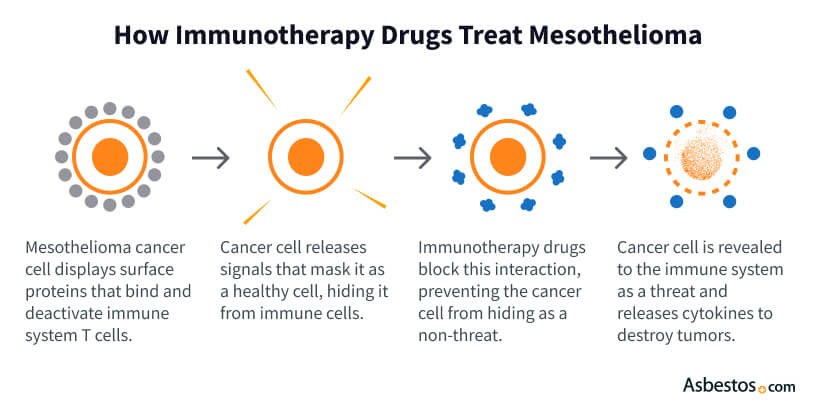
What Are the Benefits of Checkpoint Inhibitors for Mesothelioma?
Checkpoint inhibitors can help people with pleural mesothelioma that can’t be removed with surgery. They may help people live longer and shrink the tumor. The results are different for each person and can depend on things like genes and overall health. The success seen in key clinical trials led to FDA approvals.
Efficacy of Checkpoint Inhibitors for Mesothelioma
- CheckMate 743 Trial: Opdivo and Yervoy showed a median overall survival of 18.1 months versus 14.1 months for chemo alone. The 2-year survival was 41% for immunotherapy versus 27% for chemo.
- KEYNOTE-483 Trial: Keytruda combined with chemo showed a 21% reduction in risk of death and a 62% tumor response rate compared to 38% with chemo alone.
- PrE0505/DREAM Study: Imfinzi (durvalumab) plus chemo yielded a median survival of 20.4 months and a 2-year survival rate of 44%.
Checkpoint inhibitors can also help people with sarcomatoid mesothelioma. This is a mesothelioma subtype that usually doesn’t respond to chemo. In some cases, these drugs can even double survival. The type of mesothelioma you have helps doctors choose the best treatment for you.

Discover new treatments with immunotherapy clinical trials near you.
Sign Up NowWho Is Eligible for Checkpoint Inhibitor Therapy?
People need to be in good health to get checkpoint inhibitor treatment. It’s considered risky for people with autoimmune diseases. The cell type of mesothelioma you have also matters. People with the epithelioid type often respond better.
“Because the FDA has approved this as a first-line mesothelioma treatment, this is something patients can be asking their doctor about,” said Dr. Catherine Perrault, a board-certified family physician and medical officer at The Mesothelioma Center. “It’s really, really important for us to take into account the different mesothelioma cell types.”
Eligibility Criteria for Checkpoint Inhibitors
- Are in good overall health (ECOG 0-1)
- Don’t have active autoimmune diseases or organ transplants
- Haven’t previously received immunotherapy
- Have pleural mesothelioma that can’t be removed with surgery (Stage 3/4)
Checkpoint inhibitors are FDA-approved as a first-line therapy or after chemo. They’re sometimes used off-label for peritoneal mesothelioma or are available through clinical trials.
Side Effects of Immune Checkpoint Inhibitors
Checkpoint inhibitors for mesothelioma turn on the immune system to fight cancer. Because of this, they can cause side effects related to the immune system, like rash and swelling. Medications are available to manage most side effects.
Common Side Effects of Checkpoint Inhibitors
- Cough
- Diarrhea
- Fatigue
- Nausea
- Rash or skin itching
Medicines like corticosteroids can help with some side effects. Doctors can also change the treatment if needed. Patients should tell their doctors about any side effects right away.
Serious Side Effects of Immune Checkpoint Inhibitors
Serious side effects are rare. However, they can happen when mesothelioma and inflammation cause problems. For example, inflammation in the lungs can make it harder to breathe.
Rare But Serious Side Effects
- Colitis (bowel inflammation)
- Endocrinopathies (thyroid or adrenal issues)
- Hepatitis (liver inflammation)
- Pneumonitis (lung inflammation)
These side effects require early intervention. It’s important to report any new or worsening symptoms to your doctor right away. With treatment, many of these serious side effects are reversible. However, they can leave permanent damage if left untreated.
Find the top cancer centers trusted by mesothelioma patients nationwide.
Get Help NowClinical Trials Testing Mesothelioma Immune Checkpoint Inhibitors
Mesothelioma clinical trials continue to explore checkpoint inhibitors in new ways. Clinical trials let patients try new drug combinations to help fight mesothelioma better. Many of these trials are currently recruiting participants.
Active Clinical Trials for Mesothelioma
- Chemotherapy ± Atezolizumab (NCT05001880): Randomized Phase II trial adding PD-L1 immunotherapy to chemo for malignant peritoneal mesothelioma.
- DREAM3R (NCT04334759): Durvalumab plus chemo for pleural mesothelioma that can’t be removed with surgery. Phase III trial testing whether adding immunotherapy to chemo improves survival.
- NEMO Trial (NCT05932199): Durvalumab and Tremelimumab as a treatment before and after surgery for pleural mesothelioma.
- Opdivo + Yervoy for Peritoneal Mesothelioma (NCT05041062): Phase II study evaluating checkpoint inhibitors before and after surgery.
- Pembrolizumab + IMPRINT Radiation (NCT04897022): Testing a new treatment that stops PD-1 and uses radiation on the lung lining for mesothelioma that can’t be removed with surgery.
Check with your mesothelioma doctor to see if clinical trials are enrolling near you. They can help determine if you’re eligible and could benefit you.
Common Questions About Immune Checkpoint Inhibitors
- Can checkpoint inhibitors be paused or stopped if I experience severe side effects?
-
Yes. Doctors can pause or stop checkpoint inhibitor treatment if serious side effects happen. Many side effects get better with quick care, and some people can start treatment again if it’s safe.
- How will we know if the checkpoint inhibitors are working for my mesothelioma?
-
Doctors check your progress during treatment using scans like CT or PET and blood tests. If the tumor gets smaller, stays the same or grows more slowly, the treatment may be working. Your doctor will also watch your symptoms and overall health.
- Are there genetic or biomarker tests that could predict how well I’ll respond to immunotherapy?
-
Yes. Some tests check for things like PD-L1 levels or other signs in the tumor to see if treatment might work. These tests can help, but they’re not always right. Even people with low PD-L1 levels can still get good results from treatment.
- Could checkpoint inhibitors affect other conditions I have, like autoimmune disease or chronic lung issues?
-
Checkpoint inhibitors can sometimes make immune system problems worse. They can cause swelling in places like the lungs. People with these issues should talk with their cancer doctor. In many cases, treatment can be changed to help prevent serious side effects.



