Based on Your Reading:
Get Your Free Mesothelioma Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

The government plays a small role funding mesothelioma research compared to funding other cancers. Federal organizations that provide funding include the National Cancer Institute, Department of Defense, FDA-sanctioned clinical trials and the National Mesothelioma Virtual Bank.

Written by Michelle Whitmer | Edited By Walter Pacheco | Last Update: July 2, 2024
Government funding for mesothelioma is lacking because of limited awareness and relatively low incidence rates. The number of people affected by mesothelioma is small compared to other cancers. An estimated 3,000 new cases of mesothelioma are diagnosed each year.
The public’s lack of awareness about the illness plays a role. When there is less awareness of a particular disease, there is less pressure on Congress to fund it.
Doctors and scientists secure grants to fund new breakthroughs in treatment methods. Patient advocacy groups are lobbying for more funding for mesothelioma research and clinical trials. Thanks to these efforts, mesothelioma research receives more federal funding than ever before.
Funding for mesothelioma grants in 2018 paled in comparison to money allotted to other diseases. That year, the National Cancer Institute awarded the Abramson Cancer Center a $10.7 million grant to study CAR-T-cell therapy as a treatment for mesothelioma.
In contrast, breast cancer research received nearly $575 million. This cancer is given more federal funding than any other cancer. It receives more than three times the funding of lung cancer research even though its death rate is one-fourth the amount.
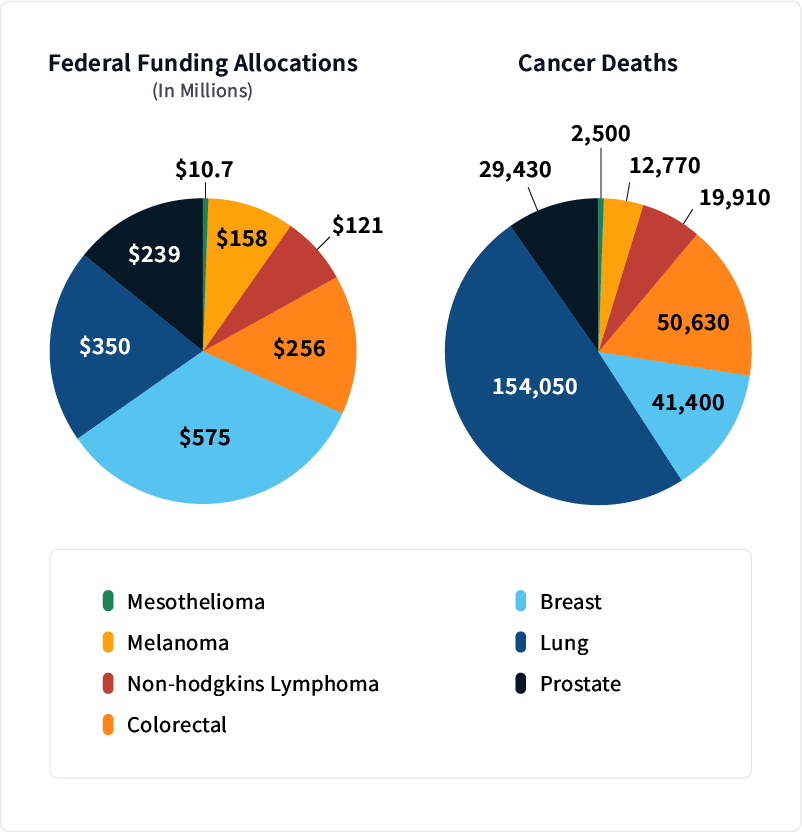
The incidences and death rates of the most common types of cancer affect funding. Greater public awareness and more intensive lobbying also play a role in the allocation of funds for diseases such as breast cancer.
The National Cancer Institute provides grants for mesothelioma research. In 2023, NCI awarded researchers at Fox Chase Cancer Center a $1.2 million mesothelioma grant to study a compound found in broccoli for the prevention of this rare cancer. NCI’s total 2024 budget is $7.22 billion.
Created in 1937, the National Cancer Institute conducts research on cancer for the federal government. It is one of 27 institutes and centers that form the National Institutes of Health. The federal Department of Health and Human Services oversees it.
The National Cancer Act of 1971 created the department. It receives its funding from Congress. The NCI investigates the causes, prevention, detection, diagnosis and treatment of cancer.
Get Your Free Mesothelioma Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

The Department of Defense has issued nearly $28 million in mesothelioma grants since activists got it to take notice of this serious disease.
In 2006, mesothelioma activists appealed to the Senate Defense Appropriations Subcommittee for the first time. This subcommittee recommends funding for the Department of Defense.
The activists convinced the subcommittee that the department should fund mesothelioma research. They persuaded the subcommittee by explaining that military personnel make up a significant portion of mesothelioma diagnoses. Activists stressed the importance of research for service members, veterans, and their dependents.
In 2019, the DoD awarded nearly $2.6 million in mesothelioma research grants. Increased funding for mesothelioma research has continued, thanks to activists who convinced the department to join the cause.
The federal government influences mesothelioma research through the sanctioning of clinical trials. The U.S. Food and Drug Administration oversees them. Clinical trials are studies of new drugs and treatments.
More than 100 mesothelioma clinical trials are recruiting participants. Over a decade ago this was not the case. The FDA has approved more trials for mesothelioma in recent years. It does not fund or conduct clinical trials on its own. Its role is to promote and enforce strict protocols to ensure that people who agree to be in studies are treated as safely as possible.
Mesothelioma clinical trials investigate new drugs and anti-cancer therapies. These trials bring researchers closer to a cure for mesothelioma. Currently, mesothelioma clinical trials primarily involve research on immunotherapy, chemotherapy, radiation and surgery. Other trials investigate gene therapy, targeted therapy and sometimes palliative care or nutritional interventions.
Recommended ReadingThank you for your feedback. Would you like to speak with a Patient Advocate?
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2024, July 2). Government’s Role in Mesothelioma Research. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/government-research/
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/mesothelioma/government-research/.
Whitmer, Michelle. "Government’s Role in Mesothelioma Research." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/mesothelioma/government-research/.
Mesothelioma Center - Vital Services for Cancer Patients & Families doesn’t believe in selling customer information. However, as required by the new California Consumer Privacy Act (CCPA), you may record your preference to view or remove your personal information by completing the form below.
