John Fiala joined a unique clinical trial using the immunotherapy combination of Opdivo (nivolumab) and Cyramza (ramucirumab). The treatment has enabled his immune system to fight the cancer in two different ways. Opdivo blocks a protein that often prevents the immune system from attacking tumors. Cyramza decreases the blood supply tumors need to metastasize.
New Mesothelioma Treatments
Some new mesothelioma treatments are giving people more hope. These include gene therapy, enzyme therapy and cancer vaccines. Some drug combinations, like immunotherapy with chemo, are also helping people live longer and feel better.
What Are the New Treatments for Mesothelioma?
New mesothelioma treatments give people more options than traditional surgery, chemotherapy and radiation. These newer therapies are tested in clinical trials to help people live longer and feel better.
Key Facts About New Mesothelioma Treatments
- Around 94 active clinical trials are testing new mesothelioma treatments or approaches.
- Many new therapies are tested in clinical trials before they become standard care.
- Some new treatments can both boost the immune system and directly target cancer cells.
- New treatments include enzyme therapy, vaccines and gene therapy.
Immunotherapy drugs Opdivo, Yervoy and Keytruda started in trials. Now they’re approved as first-line treatments. Some treatments are completely new, while others improve how existing therapies are used.
For example, ADI-PEG20 (pegargiminase) is an enzyme therapy doctors have studied for about 20 years. But in a 2024 clinical trial, patients who took ADI-PEG20 with chemotherapy lived 4 times longer after 3 years than those who got only standard chemo. This was a major step forward for people living with mesothelioma.
New Immunotherapy and Targeted Therapy for Mesothelioma
Immunotherapy became an approved first-line treatment for pleural mesothelioma in 2024. Once considered experimental, it continues to evolve as researchers test new immunotherapy drugs and combinations with other therapies.
Some treatments, like Keytruda, Opdivo and Yervoy, are both immunotherapy and targeted therapy. These drugs are checkpoint inhibitors and monoclonal antibodies. They help the immune system find and attack cancer. They also target specific parts of the cancer cells.
Targeted therapy is showing strong promise for mesothelioma. It targets only cancer cells, not healthy cells. Targeted therapies focus on the genes or proteins in mesothelioma cells. This helps make treatment more personal and, in some cases, more effective.
Enzyme Therapy
ADI-PEG20 enzyme therapy did well in the ATOMIC-Meso clinical trial. Now, researchers are testing other enzyme therapies. One trial is testing the targeted therapy olaparib.
Olaparib is considered a type of enzyme therapy called enzyme inhibition therapy. It blocks PARP enzymes, which cancer cells use to repair their DNA. When these enzymes are blocked, the cancer cells can’t fix themselves and eventually die.
Epigenetic Therapy
Epigenetic therapy is a type of targeted therapy. It changes how genes turn on or off without changing the DNA itself. This can slow cancer growth. One clinical trial is studying Tazverik (tazemetostat) for mesothelioma. This drug is already used to treat some soft tissue cancers, like those in muscles and nerves.
As Sean Marchese, RN, explains, “Some of the most exciting treatments for mesothelioma involve changing DNA or genes. Genomic sequencing is going to be the future of personalized medicine.”
Gene Therapy
Gene therapy takes the introduction of genetic information a step further. It alters genetic information to boost the immune system or fight mesothelioma cells directly.
Active clinical trials exist for “suicide gene therapy” and BAP1 gene therapy. Suicide gene therapy is a cancer treatment that uses genes to induce the death of target cells. Gene therapy can fix a broken BAP1 gene to stop tumors from growing. Some treatments use “suicide genes” inside cancer cells to help chemotherapy work better.
Mesothelin-targeting therapies include monoclonal antibodies, immunotoxins, antibody-drug conjugates, vaccines and CAR-T therapy. These treatments focus on mesothelin proteins found on mesothelioma cells. They’re both immunotherapy and targeted therapy.
Examples of Mesothelin-Targeted Therapies
- ADCs
- CAR T-cell therapies
- Immunotoxins
- Monoclonal antibodies
- Therapeutic vaccines
Clinical trials are testing new mesothelioma treatments to see if they’re safe and work well. These trials provide hope of finding a mesothelioma cure. If they work well, doctors may use these new treatments to help people live longer and feel better.
Find the top cancer centers trusted by mesothelioma patients nationwide.
Get Help NowVaccine Therapy
Cancer vaccines help the immune system find and attack mesothelioma cells. The UV1 vaccine has shown good early results and earned fast-track status from the FDA. A recent study tested UV1 with Opdivo and Yervoy. It helped almost twice as many patients respond to treatment.
Studies of the galinpepimut-S vaccine also had positive results for people with mesothelioma. The GPS vaccine is an immunotherapy targeting a protein called WT1. It doesn’t prevent cancer like traditional vaccines, but trains the body to fight tumor cells. The GPS vaccine stabilized cancer in one-third of patients, shrinking tumors 17%.
Virotherapy
Virotherapy uses modified viruses to attack and kill mesothelioma cells. These viruses also help the immune system notice the cancer. Different types of virotherapy are being tested in clinical trials to see how well they work.
Photodynamic Therapy
Photodynamic therapy uses a special drug that collects in cancer cells. A doctor then shines a laser light to turn the drug on and help kill the cancer. The activated drug kills the cells and can stop the cancer from spreading.
This treatment is promising because it causes few side effects. This means patients can have a better quality of life while getting treatment. Researchers tested low-dose photodynamic therapy (L-PDT) along with immunotherapy on mice. In mouse models, the results showed this treatment completely got rid of mesothelioma in 37.5% of the mice tested.
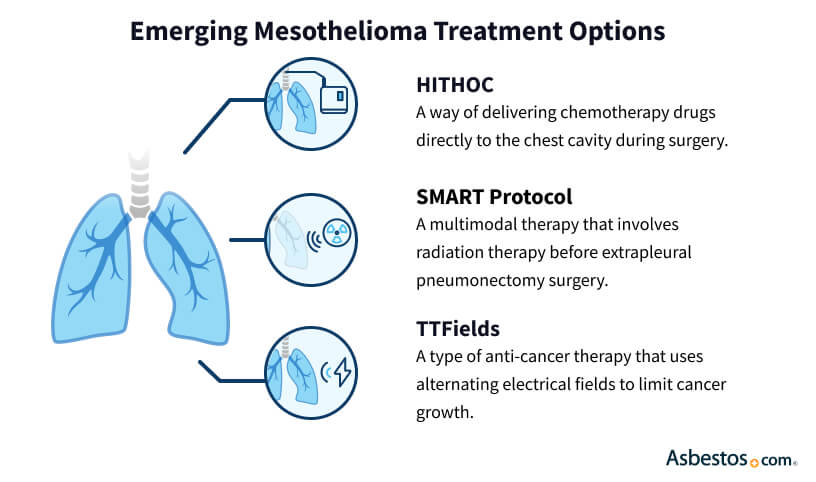
Multimodal Therapy: Combining Mesothelioma Treatments
Multimodal therapy is a combination of already established treatments and emerging mesothelioma treatments. For example, multimodal therapy uses surgery first then radiation. This helps kill cancer cells left after surgery. However, patients receiving multiple therapies also experience more side effects.
Some combinations that were once experimental are now part of standard mesothelioma care. Combining Keytruda and chemo and TTFields with chemo are now both standard.
How to Access New Mesothelioma Treatment
You may be able to get new mesothelioma treatments in clinical trials or from your mesothelioma doctor. These treatments are tested at top cancer centers. To join a trial, you must meet certain health and safety rules.
Snehal Smart, MD, a Patient Advocate, tells us, “Many patients ask us about clinical trials for new mesothelioma treatments. We can help guide them to these types of resources.”
Ways to Receive New Mesothelioma Therapies
- Join a clinical trial for mesothelioma to try a new treatment before it becomes widely available.
- Work with a mesothelioma specialist who knows about the latest treatment options.
- Get care at a mesothelioma cancer center where trials are often available.
- Talk to a Patient Advocate who can help you find trials and treatment centers
Eligibility depends on cancer stage, tumor location, health and past treatments. Getting access to new treatment starts with the right care team. Working with a specialist or a Patient Advocate can help you find clinical trials that suit you.

Discover how mesothelioma doctors personalize treatment plans for you.
Sign up nowHow Does the FDA Approve New Mesothelioma Treatments?
The FDA must approve all new mesothelioma treatments before doctors can use them. This helps make sure the treatments are safe and work well. The process starts with lab tests and ends with a full FDA review.
FDA-Approval Process for Mesothelioma Therapies
- Preclinical testing: Scientists test the drug in labs and on animals to check safety.
- IND application: Drug makers ask the FDA for permission to test the drug in people.
- Clinical trials (phases 1–3): The drug is tested in people to learn if it is safe and helpful.
- New drug application: After testing, drugmakers send all results and safety info to the FDA.
- FDA review and monitoring: Experts check the data and decide if the drug can help patients. If approved, the FDA keeps watching how the drug works in real life.
The FDA may speed up this process for rare cancers like mesothelioma. Even after approval, the FDA continues to check that the drug stays safe and works as expected.
New Research for Treating Mesothelioma
Researchers use AI and computers to find the best treatments and predict how people will respond. Virtual reality helps doctors plan complex surgeries to improve results.
New ideas include diet changes to support treatment and give medicines in less painful and easier ways. These advancements could change the face of mesothelioma treatment.
Scientists have also examined capsaicin, a natural substance found in chili peppers. They found that it helps stop mesothelioma cancer cells from growing and moving.
Common Questions About Emerging Mesothelioma Treatments
- How does a new mesothelioma treatment differ from conventional treatments?
-
New mesothelioma treatments differ from standard ones because they’re still being tested. They might offer unique benefits for patients who otherwise have few options.
- Am I a candidate for new mesothelioma treatments?
-
People with mesothelioma may qualify for new treatments, depending on their cancer stage, type and health. It’s important to consider the benefits and risks of any treatment or trial. A mesothelioma doctor or Patient Advocate can help find the best trials for you.
- Is there financial assistance to help me afford new mesothelioma treatments?
-
Clinical trial sponsors fund new and emerging mesothelioma treatments during testing. Financial assistance like travel grants, reimbursements and waivers can also help.
- What is the latest treatment for mesothelioma?
-
The latest treatments for mesothelioma in research include targeted, vaccine and gene therapies. They also include new combinations of immunotherapy treatments.
- How long does it take for a new mesothelioma treatment to become available?
-
It can take years to get a mesothelioma treatment once it’s tested and FDA-approved. Safety must first be established through rigorous testing and evaluation.







