Get in Touch
Have questions? Call or chat with our Patient Advocates for answers.
Mesothelioma blood tests detect proteins, called biomarkers, linked to mesothelioma. These tests may detect n-ERC mesothelin and fibulin-3. Doctors use these tests with imaging scans and biopsies to confirm mesothelioma. The MESOMARK assay is the only FDA-approved mesothelioma blood test.
Written by Dr. Kristopher Bunting | Edited By Walter Pacheco | Last Update: July 23, 2024
Blood tests for mesothelioma aim to detect substances cancer cells produce, called biomarkers. Currently, blood tests alone are not accurate enough to diagnose mesothelioma.
While a direct blood test for mesothelioma does not yet exist, blood tests can show possible indicators of cancer. Instead of relying solely on mesothelioma blood tests, doctors also use detailed imaging scans and tissue biopsies. By combining these results, doctors can better confirm a diagnosis of asbestos-related illnesses.
Mesothelioma survivor Chris Shelton discovered he had cancer while hospitalized for more than a month after a severe motorcycle accident. He had many blood tests during this time to monitor his recovery and overall health. Eventually, after much more testing, Shelton received a mesothelioma diagnosis.
The doctor came into my hospital room and said he had good news and bad news. He said, ‘You’ll walk again, but your blood work makes us think you have some sort of cancer.’
No blood test can detect asbestos exposure. Biopsy tests of tissues, however, can reveal the presence of asbestos fibers that can get stuck in tissues. Doctors often use biopsies to determine a diagnosis of mesothelioma.
Mesothelioma blood tests screen for biomarkers, proteins and other substances found in blood and tissue that can indicate health statuses. For example, blood sugar and hemoglobin A1C are useful indicators to diagnose diabetes and monitor treatment. Cancer cells produce certain proteins that circulate in the blood.
For some tumors, biomarker proteins are unique to that specific cancer. Identifying them in a blood test indicates a person may have that cancer. The biomarkers for mesothelioma are also present in healthy people but are higher when mesothelioma exists. However, these tests are not always accurate and are not definitive proof of a diagnosis.
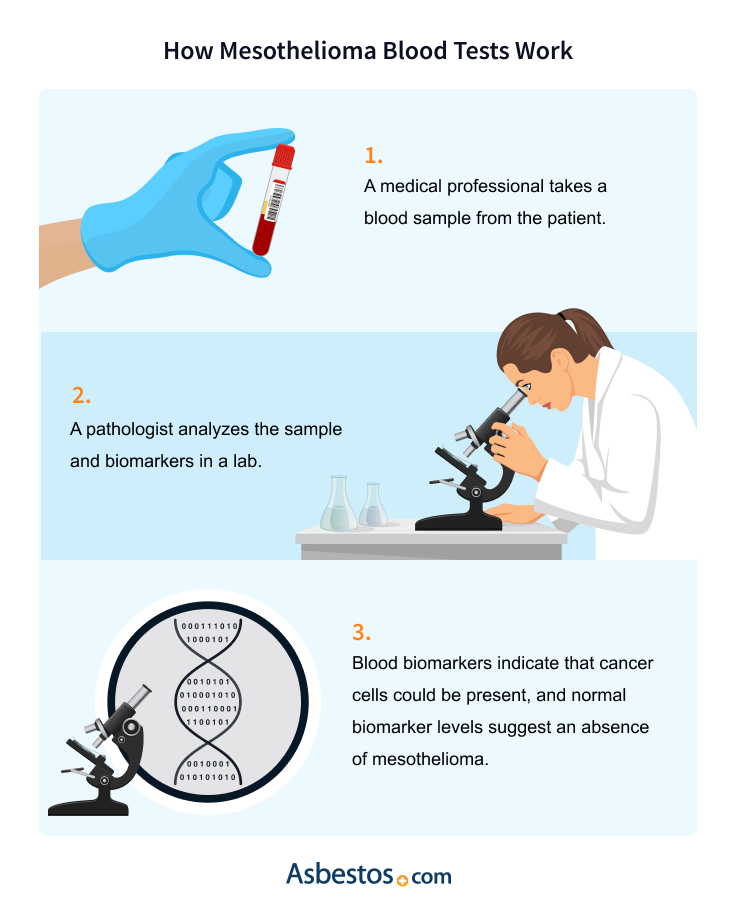
Blood tests can help confirm a mesothelioma diagnosis and rule out other cancers. Many cancers originate in the lungs or spread to the lungs from other parts of the body. Different cancers express different immunohistochemical markers, proteins that labwork can identify using antibodies.
There are no biomarkers specific to mesothelioma, but levels of the proteins calretinin, cytokeratin 5 and 5/6, WT-1, podoplanin (D2-40) and mesothelin help identify possible mesothelioma.
Dr. Snehal Smart of The Mesothelioma Center explained that blood tests can “help find mesothelioma in a patient before symptoms even begin.” Blood tests such as MESOMARK look for a particular protein – the SMRP protein.
Smart said this protein is “usually high in patients with mesothelioma.” Therefore, while a blood test such as a MESOMARK assay cannot diagnose a patient independently, “high levels of the SMRP protein can indicate a probable diagnosis of mesothelioma.”

Gain access to top mesothelioma doctors and get help scheduling appointments.
Connect NowThe MESOMARK assay, N-ERC/mesothelin test and fibulin-3 test are three blood tests that can help doctors accurately diagnose mesothelioma. Each test has strengths and weaknesses, but they can help doctors accurately diagnose mesothelioma when used with biopsies and imaging studies.
A blood test must be sensitive and/or specific for it to be useful for diagnosis. A test with high sensitivity is better at detecting mesothelioma, and a test with high specificity can help rule out mesothelioma. Some tests are more sensitive than specific, and vice versa.
For example, the N-ERC/mesothelin test is very sensitive but not specific. When N-ERC/mesothelin levels are high, there’s a high likelihood that mesothelioma is present. However, a person may still have mesothelioma even if their N-ERC/mesothelin levels are low.
The fibulin-3 test, on the other hand, is very specific for mesothelioma. If fibulin-3 levels are low, there is little chance of mesothelioma. In other words, the N-ERC/mesothelin test can usually detect mesothelioma, while the fibulin-3 test can generally tell if you don’t.
The performance of all of these blood tests requires a technique called ELISA. Medical professionals collect a blood sample and send it to the laboratory, mixed with antibodies that target a specific biomarker. Using sophisticated equipment, the lab can determine the levels of biomarkers in the blood.
The MESOMARK assay detects most types of mesothelioma and measures the effectiveness of treatment.
The MESOMARK assay measures serum-measured soluble mesothelin-related peptides (SMRP), proteins made by mesothelial cells. Healthy cells produce low levels of SMRP, but high SMRP levels may indicate the presence of mesothelioma. As treatment progresses, SMRP levels decrease.
A MESOMARK blood test is performed by the physician or nurse practitioner drawing a sample of blood. That blood is sent to a lab where the levels of SMRP protein are measured.
Some types of mesothelioma, such as sarcomatoid tumors, do not release high levels of SMRP. Because of this, the U.S. Food and Drug Administration recommends that doctors combine the MESOMARK assay with other tests to ensure an accurate mesothelioma diagnosis.
Some insurance companies do not cover this blood test. If you have a history of asbestos exposure, ask your doctor if the MESOMARK blood test would be helpful.
The N-ERC/mesothelin test detects a specific form called N-ERC/mesothelin. Similar to MESOMARK, it looks for a specific version of the mesothelin molecule, which increases its accuracy.
The test is 95% effective at detecting mesothelioma. However, because several other types of cancer also increase mesothelin levels, it is only 76% effective at ruling out mesothelioma. This test is more accurate than MESOMARK but, like other blood tests, cannot be used to diagnose mesothelioma independently.
Mesothelioma cells produce a protein called fibulin-3. Specific tests can detect this protein in pleural fluid and blood. Mesothelioma specialist Dr. Harvey Pass helped develop it for diagnosis.
He co-authored a study showing that the test is 96.7% effective at detecting mesothelioma and 95.5% effective at ruling it out in people without the disease.
Several researchers have found that the sensitivity of the fibulin-3 test can vary. This variation potentially makes the test less effective for detecting mesothelioma. However, it is still highly effective in ruling out mesothelioma.
Research also shows that fibulin-3 levels are higher in people with known asbestos exposure. This result means the fibulin-3 test is very good at determining whether an individual has had significant asbestos exposure.
Additionally, fibulin-3 levels can help predict mesothelioma prognosis. Very high levels indicate a worse prognosis. Fibulin-3 testing is beneficial when combined with other mesothelioma blood tests.
As researchers discover more about mesothelioma, they find new biomarkers that may improve its diagnosis and treatment. New biomarker tests can help medical professionals diagnose mesothelioma, distinguish it from other cancers, predict disease prognosis, select the most effective treatment and monitor treatment progression.
Discovering new biomarkers is essential in understanding how mesothelioma develops. Some biomarkers are proteins involved in cells becoming cancerous, such as YAP, a protein that interacts with DNA. These biomarkers can reveal new targets for therapies, such as immunotherapy.
Some of these tests are available, but others are still in development. Health care professionals may eventually use some of these tests to screen for mesothelioma in people who have not yet developed any symptoms. Screening tests can help improve outcomes by detecting cancer early, allowing for early treatment.
When you talk to your doctor about mesothelioma, ask about available tests. Whether seeking a diagnosis, considering treatment options or undergoing treatment, using newer biomarker tests can help you and your doctor make better decisions.
Recommended ReadingThank you for your feedback. Would you like to speak with a Patient Advocate?
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Bunting, K. (2024, July 23). Mesothelioma Blood Tests and Biomarkers. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/mesothelioma/blood-test/
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com, 23 Jul 2024, https://www.asbestos.com/mesothelioma/blood-test/.
Bunting, Kristopher. "Mesothelioma Blood Tests and Biomarkers." Asbestos.com. Last modified July 23, 2024. https://www.asbestos.com/mesothelioma/blood-test/.
Mesothelioma Center - Vital Services for Cancer Patients & Families doesn’t believe in selling customer information. However, as required by the new California Consumer Privacy Act (CCPA), you may record your preference to view or remove your personal information by completing the form below.
