Get Your Free Mesothelioma Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

Talc and asbestos are naturally occurring minerals, but only asbestos is a known carcinogen. Talc has been used in cosmetics, industrial products and personal hygiene products, including Johnson’s Baby Powder. Asbestos-contaminated talc can cause malignant mesothelioma and ovarian cancer.

Talcum powder is made of talc, a soft mineral composed of hydrated magnesium silicate. Talc can be green, white or gray and has a texture like soap.
Manufacturers grind talc down until it’s a fine powder that absorbs moisture and reduces friction. Talcum powder became popular as an ingredient in baby powder, cosmetics and deodorant. It is used for its ability to prevent chafing and skin rashes, as well as disguise odor.
For more than 100 years, Johnson & Johnson has been the most famous name in talcum powder, mainly through its line of baby powders. Men and women of all ages use baby powder and other talc-containing products routinely.
Court documents show J&J officials knew asbestos had been in its popular Johnson’s Baby Powder product since the 1950s. In 2023, the company stopped selling talc in its products worldwide. J&J was facing over 61,000 asbestos lawsuits in August 2024.
Talc dust can irritate the lungs. It can cause coughs, chest pain and shortness of breath. Researchers at Mount Sinai Hospital say breathing in talcum powder can lead to lung problems or death. This applies to pure talcum powder and asbestos-contaminated talcum powder.
The World Health Organization says pure talc is “probably carcinogenic.” They say genital (perineal) use of pure talc has limited evidence for causing ovarian cancer. The organization also classifies asbestos-contaminated talc as definitely carcinogenic to humans. Some consumer and industrial products have contained dangerous levels of asbestos-contaminated talc.
Oftentimes when you ask somebody who didn’t really have any traditional exposure to asbestos, if they regularly used talc products, the answer is often yes.

Both the National Institutes of Health and the WHO’s International Agency for Research on Cancer released talc reports in 2024. They covered the potential health risks of talcum powder. A May 2024 NIH report found an increased risk of ovarian cancer among heavy talcum powder users. In July 2024, the IARC declared pure talc as probably carcinogenic.
The NIH’s study looked at more than 50,000 women who frequently used talcum powder. The IARC classified talc in Group 2A. It is its “second highest” cancer risk level. This is based on evidence of ovarian cancer in humans. The IARC also reported strong lab evidence that talc is carcinogenic to human cells.
Health Risks of Talcum Powder
Both pure talcum powder and asbestos-contaminated talc are associated with serious medical conditions. Those at risk include users of talcum powder and workers exposed to talc during its production or industrial use. Parents shouldn’t use talcum powder on babies. It can cause fatal aspiration. After using baby powder and developing cancer, thousands of women filed lawsuits against J&J and others. These claims allege that repeated use of talc led to ovarian cancer.
Get Your Free Mesothelioma Guide

Find a Top Mesothelioma Doctor

Access Help Paying for Treatment

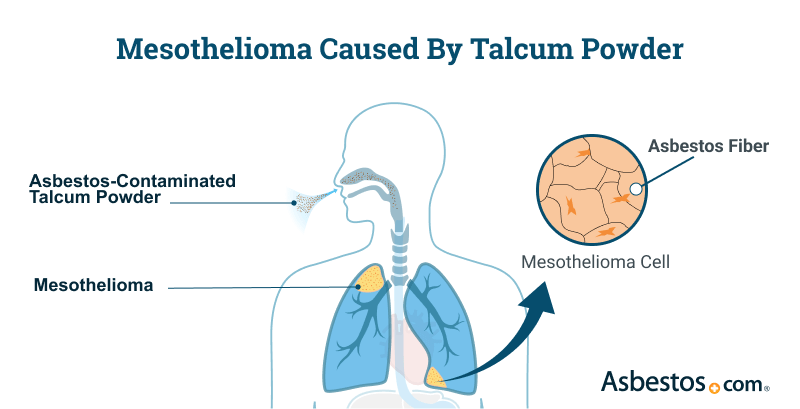
Talc with asbestos can cause mesothelioma and other asbestos cancers. Malignant mesothelioma has a latency period of 20 to 60 years.
The risk of developing an asbestos disease like mesothelioma from exposure to contaminated talc varies. It depends on the duration and amount of exposure. The risk is dose-dependent. It increases with longer, higher levels of asbestos exposure. Long-term exposure to low-level asbestos concentrations can cause cancer. So can short-term exposure to high-level concentrations.
Talc and asbestos can naturally form so closely together that mining practices cannot keep them separated. This fact has been documented in geology books as early as 1872.
Not every talc deposit contains asbestos. But most are contaminated with it. Talc deposits tend to contain the most toxic forms of asbestos, such as tremolite or anthophyllite. These forms are more carcinogenic than chrysotile, the most-used type of asbestos.
Higher levels of contamination carry a higher risk of mesothelioma. Different grades of talc have different amounts of asbestos. Industrial talc has the highest asbestos levels reaching 50% to 70%. Since the late 1960s, tests of cosmetic talc found asbestos levels from 0% to 30%.
Asbestos-Contaminated Talc Research
Finding asbestos-free talc deposits throughout the world has been challenging for manufacturers. A J&J company memo from 1969 unsealed through litigation said it was normal for tremolite asbestos to occur in U.S. talc deposits. By this time, the company had spent more than a decade purchasing talc mines throughout the world in search of a pure source.
Children, adult consumers and workers in specific industries remain at risk of exposure to asbestos-contaminated talcum powder. Tests as recent as 2018 and as far back as 2000 have revealed asbestos-contaminated talcum powder in kid’s toys and makeup. In 2024, testing showed asbestos in Dynacare brand baby powder.
Anyone who has used talc consistently for many years, or has a known history of asbestos exposure, should talk with their doctor at the first sign of any respiratory symptoms. Also, you may want to see a mesothelioma specialist or a pulmonologist who treats asbestos diseases.
Hairdressers, barbers and their family members are at risk of exposure to cosmetic-grade talcum powder. It is used to prevent chafing and irritation after haircuts.

Talc is a filler in ceramics. It improves thermal shock resistance. This gives strength after firing.

Contaminated toys have included crayons, modeling clay and amateur crime lab kits. Contaminated children’s makeup was sold at Justice and Claire’s stores.

Adults are at risk of contaminated talcum powder. It mainly comes from cosmetics and personal care products. These include makeup, body powders and shaving products.

In paint, talc offers a layer of weather and corrosion resistance.

Mine and mill workers likely face the highest exposure to raw talc and nearby asbestos. They also wear protective equipment to reduce exposure.

J&J announced it would halt all sales of talc-based baby powder worldwide in 2022. This was a reversal of the company’s previously announced plan to continue international sales of talc-based baby powder because demand remained high.
The company had already announced it would stop selling its talc-based baby powder in the U.S. and Canada in May 2020. J&J said it was switching to a cornstarch-based version of its baby powder instead. The company claimed the decision to change was the result of a decline in sales and “misinformation” around the product’s safety.
Ending sales of its talc-based baby powder followed a contamination scandal. The U.S. Food and Drug Administration found asbestos in a bottle of Johnson’s Baby Powder in 2019. This report forced the company to issue a recall of the batch associated with the contaminated bottle.
Talc products containing asbestos have included personal hygiene products, cosmetics, children’s toys and industrial talc products. Talc is a common ingredient in many home and industrial goods. The most widely used consumer talc product is talcum powder.
Companies With Asbestos-Contaminated Talc Products
Between 1948 and 2017, 66% of 1,032 cosmetic talc products tested through litigation were positive for asbestos. Asbestos contamination in talc products has varied. Cosmetics had trace amounts. Some industrial products, like Nytal, had higher than 50%.
Not every talc product contains asbestos, but it’s impossible to detect on your own. Only testing can reveal if a product is contaminated. The FDA’s follow-up testing of cosmetic talc products in 2021 and 2022 revealed no asbestos in 100 samples.
In addition to talcum powder, cosmetic-grade talc is in many cosmetic products. Examples include foundation, creams and moisturizers, eye shadow, blush and mascara. Cosmetic-grade talc is approximately 98% pure talc.
Talc-Containing Cosmetics With Asbestos
In November 2020, the Environmental Working Group found asbestos-contaminated talc in 3 out of 21 cosmetics samples tested. The FDA found asbestos in 9 out of 52 cosmetics samples in 2019.
In 2017 and 2018, several cases of talc contamination involved children’s makeup. National retailers Justice and Claire’s sold it. Other children’s toys containing asbestos-contaminated talc have included crayons, modeling clay and crime-scene kits.
We have done the investigation and know the companies who use talcum powder contaminated with asbestos who have led to this terrible disease. If you were diagnosed with mesothelioma and you use talcum powder in your life, you may have a case, which is why you need to speak with a mesothelioma attorney.
Industrial talc is used in many modern products, like paint and glazes. It improves texture, matting and adhesion. The paper industry uses talc to improve print quality and reduce friction. Wastewater treatment plants also use talc to purify water.
Industrial Talc Products
Industrial-grade talc contains other minerals. Their amounts vary by source. For example, the industrial talc product used in ceramics known as Nytal 100 contained 30% talc, 40% tremolite, 20% serpentine chrysotile and 10% anthophyllite asbestos.
Plaintiffs diagnosed with asbestos disease who were exposed to contaminated talc are filing talc lawsuits. According to KCIC’s most recent industry report, Asbestos Litigation: 2024 Year in Review, of the 3,931 total asbestos filings in 2024, 19.1% alleged talc exposure. This was a 17.9% increase in the number of talc filings from 2023.
Courts have awarded millions of dollars in recent talc lawsuits. Records revealed in lawsuits show some manufacturers and talc suppliers were aware of asbestos contamination and tried to cover it up. Examples include Vanderbilt Minerals, Imerys Talc America and Whittaker, Clark & Daniels. In one example, Imerys blended its talc with other sources of talc to dilute the asbestos concentration.
Asbestos-Contaminated Talc Verdicts
Many companies sourced their talc from asbestos-contaminated mines, including sites in New York, North Carolina, Alabama, Vermont and northern Italy. Some of the products sold in the U.S. that tested positive for asbestos, including children’s makeup sold in Claire’s stores, were made in China, which has no regulations for asbestos or talc.
Compensation is available through a personal injury lawsuit. People who develop mesothelioma from exposure to asbestos-contaminated talcum powder qualify to file. If you’ve lost a loved one to an asbestos-related disease, you may be eligible to file a wrongful death claim. Settlements resolve most of these lawsuits, and others get resolved through a jury trial.
In April 2025, United States Bankruptcy Judge Christopher M. López of the Southern District of Texas denied J&J’s third attempt at declaring bankruptcy and its proposed $10 billion baby powder settlement. In October 2021, J&J created a subsidiary to absorb its talc liabilities. The recent proposed settlement aimed to end more than 60,000 ovarian cancer lawsuits. However, it wouldn’t have impacted mesothelioma cases. J&J claims it has already settled 95% of all mesothelioma lawsuits against the company.
In June 2024, J&J offered to pay $700 million to settle an investigation 42 U.S. states and Washington, D.C. State officials initiated. They accused J&J of misleading marketing practices claiming its talc products, including Johnson’s Baby Powder, were safe. The agreement resolves accusations that J&J misled consumers. The company admits no wrongdoing and denies violating the law.
Asbestos lawsuits against J&J have cost the company billions in settlements and jury verdicts. It has spent roughly $1 billion in legal defense and $3.5 billion on plaintiff awards.
J&J settled roughly 1,000 talc lawsuits in October 2020 for more than $100 million. The Missouri Court of Appeals ordered the company in June 2020 to pay $2.1 billion to 22 women who claimed Johnson’s Baby Powder caused ovarian cancer. The company also paid out millions in individual lawsuits, including $325 million in 2019 to Donna Olsen. She claimed she developed mesothelioma after using Johnson’s Baby Powder.
Residents of the United Kingdom began filing talc lawsuits in U.S. courts in 2019. Rulings from these cases determined that U.S. courts are the best venue for U.K. residents’ talc lawsuits. U.K. women have received multimillion-pound awards since these rulings.
In 2019, Hanna Louise Fletcher filed the first U.K. talc lawsuit in New York City courts. It claimed she developed mesothelioma at the age of 45. The lawsuit said she used her mother’s talc products as a child. It also claimed she bought contaminated talc products in the U.S. while on vacation.
The judge ruled that the case should remain in New York because it’s the defendants’ principal place of business. The fact that the plaintiff purchased products in New York also played a role in the judge’s ruling.
For many years a lot of people didn’t realize that cosmetic talc products were contaminated with asbestos. So being aware of all the different cosmetic talc products that contain asbestos is sort of the newest frontier of exposures that nobody had ever really discovered in the past.

Many companies, including J&J, claim that talc is safe for cosmetic and personal hygiene products. However, recent research and multiple cancer organizations warn that contaminated talcum powder can increase the risk of mesothelioma, ovarian cancer and other malignancies. Researchers at Mount Sinai Hospital caution that breathing in uncontaminated talcum powder can lead to lung problems or death.
The best way to protect yourself from asbestos in talc is to not use talc-containing products. If you must use talc but are worried about asbestos, follow the Occupational Safety & Health Administration guidelines for asbestos workers. They will reduce your exposure. For example, protections include using a respirator mask with a HEPA-filter and wearing disposable clothing, shoe covers and goggles.
The most common alternative to talcum powder is cornstarch. It is widely available in grocery stores, drugstores, and online. Cornstarch provides many of the same benefits as talcum powder without the risk of cancer. Other alternatives include baking soda, tapioca starch, rice starch and oat flour.
Talc and asbestos are minerals that naturally form close to one another. Talc deposits are often contaminated with asbestos. They are difficult to separate, which is how asbestos ends up in the final product.
Asbestos in contaminated talcum powder can be inhaled while applying the product. If used on the genitals, asbestos can travel inside the body. Once inside the body, asbestos fibers travel to sensitive tissues in the pelvis, chest and abdomen. They slowly cause diseases such as mesothelioma and ovarian cancer.
Asbestos in talc causes mesothelioma. Affected patients can file an asbestos claim. A mesothelioma lawyer helps patients get compensation for medical costs and related expenses.
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Whitmer, M. (2025, June 27). Talcum Powder and Asbestos. Asbestos.com. Retrieved July 15, 2025, from https://www.asbestos.com/products/talcum-powder/
Whitmer, Michelle. "Talcum Powder and Asbestos." Asbestos.com, 27 Jun 2025, https://www.asbestos.com/products/talcum-powder/.
Whitmer, Michelle. "Talcum Powder and Asbestos." Asbestos.com. Last modified June 27, 2025. https://www.asbestos.com/products/talcum-powder/.
Dr. Jeffrey Velotta is an experienced thoracic surgeon and pleural mesothelioma specialist at Kaiser Permanente Oakland Medical Center in California. Velotta also serves as an assistant professor at the University of California, San Francisco.
Our fact-checking process begins with a thorough review of all sources to ensure they are high quality. Then we cross-check the facts with original medical or scientific reports published by those sources, or we validate the facts with reputable news organizations, medical and scientific experts and other health experts. Each page includes all sources for full transparency.
Please read our editorial guidelines to learn more about our content creation and review process.
