Get in Touch
Have questions? Call or chat with our Patient Advocates for answers.
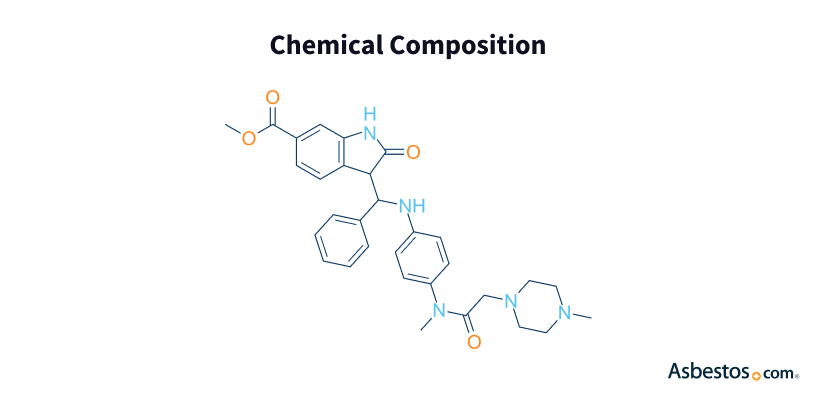
Nintedanib shows promise as an addition to the standard chemotherapy regimen for pleural mesothelioma. The drug is already approved for treating pulmonary fibrosis, and it is being tested in clinical trials for mesothelioma. Nintedanib is marketed under the brand name Ofev.
Written by Karen Selby, RN | Medically Reviewed By Dr. Rupesh Kotecha | Edited By Walter Pacheco | Last Update: June 27, 2024
The U.S. Food and Drug Administration (FDA) first approved Ofev (nintedanib) in 2014 as a treatment for progressive lung scarring. In Europe, a version of the drug named Vargatef was also approved for use in chemotherapy for lung cancer.
In 2016, the FDA granted Ofev orphan drug designation for mesothelioma in response to promising phase II clinical trial results.
This designation encourages drug development for rare diseases. Other clinical trials of Ofev for mesothelioma are ongoing.
Ofev blocks tyrosine kinase, an enzyme that triggers scar-tissue growth and blood-vessel development.
Blocking tyrosine kinase also reduces tumor growth and promotes tumor-cell death in certain types of cancer.
Ofev is approved in the U.S. to treat idiopathic pulmonary fibrosis, a disease where lung scarring makes breathing progressively harder. The drug can slow down the buildup of scar tissue in the lungs.
Asbestosis is a type of pulmonary fibrosis, so Ofev is a potential treatment for asbestosis.
| Ofev Information | |
|---|---|
| Name | Ofev |
| Alternate Names | Nintedanib, Vargatef |
| Manufacturer | Boehringer Ingelheim |
| Dosage | 200 mg twice daily |
| Administration Route | Oral |
| Active Ingredient | Nintedanib |
| Drug Class | Tyrosine Kinase inhibitor |
| Medical Code | BIBF 1120 |
| Related Drug | Pirfenidone |
| Interacting Drug | Erythromycin, rifampicin, carbamazepine, phenytoin, St. John’s wort, warfarin, carbamazepine |
| Medical Studies | Nintedanib Plus Pemetrexed/Cisplatin in Patients With Malignant Pleural Mesothelioma: Phase II Results From the Randomized, Placebo-Controlled LUME-Meso Trial |
| FDA Warning | Liver problems, gastrointestinal disorders, fetal toxicity, cardiovascular events, bleeding problems, gastrointestinal perforation |
A 2018 Clinical Cancer Research study explains how Ofev may also be effective against malignant pleural mesothelioma.
Laboratory experiments showed that Ofev inhibits mesothelioma cell growth and prevents tumors from growing blood vessels. The study results suggested Ofev may work better than bevacizumab, a similar drug being tested in mesothelioma chemotherapy.

Try our new clinical trials search tool to find active trials near you. Get help enrolling today.
Find a Clinical TrialOfev has reached phase III in the clinical trial process. If it continues to show strong results in the coming years, the FDA may add it to the standard of care for mesothelioma.
In 2013, cancer centers worldwide began to test Ofev for mesothelioma as part of phase II and III clinical trials called LUME-Meso.
The trial recruited pleural mesothelioma patients with the epithelioid or biphasic cell type. The patients could not be eligible for surgery or have already received chemotherapy.
Researchers randomized the trial participants into two groups. One group received standard chemotherapy plus Ofev, while the other received chemotherapy plus a placebo. LUME-Meso is a double-masked trial, meaning neither patients nor their doctors knew who received a placebo.
By 2016, the combination had proven safe, and the trial had proceeded to phase III. Its goal is to confirm that the combination is more effective than standard chemotherapy alone.
The Phase II findings of this trial showed promise. A 2017 article in the Journal of Clinical Oncology detailed the outcomes for 87 participants.
Median survival was 20.6 months for epithelioid patients receiving Ofev, compared to 15.2 months for those receiving placebos.
Patients on Ofev were more likely to experience low white blood cell counts, but serious side effects were actually less common overall in the experimental group.
Though the LUME-Meso trial is no longer recruiting, pleural mesothelioma patients may be eligible for other clinical trials involving Ofev.
The most common side effects of Ofev are gastrointestinal problems such as diarrhea, nausea and abdominal pain. Patients should alert their doctor immediately if they experience severe stomach pain, vomiting, sudden dizziness or bleeding problems.
Women who are pregnant or breastfeeding should avoid taking Ofev. The drug is also not recommended for people with liver problems.
Recommended ReadingThank you for your feedback. Would you like to speak with a Patient Advocate?
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Nintedanib for Mesothelioma. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/ofev/
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/ofev/.
Selby, Karen. "Nintedanib for Mesothelioma." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/ofev/.
A medical doctor who specializes in mesothelioma or cancer treatment reviewed the content on this page to ensure it meets current medical standards and accuracy.
Please read our editorial guidelines to learn more about our content creation and review process.
Dr. Rupesh Kotecha is a renowned radiation oncologist in leadership roles at Miami Cancer Institute. He is an associate professor at Florida International University's college of medicine and an adjunct faculty member at Memorial Sloan Kettering.
A medical doctor who specializes in mesothelioma or cancer treatment reviewed the content on this page to ensure it meets current medical standards and accuracy.
Please read our editorial guidelines to learn more about our content creation and review process.
Dr. Rupesh Kotecha is a renowned radiation oncologist in leadership roles at Miami Cancer Institute. He is an associate professor at Florida International University's college of medicine and an adjunct faculty member at Memorial Sloan Kettering.
Mesothelioma Center - Vital Services for Cancer Patients & Families doesn’t believe in selling customer information. However, as required by the new California Consumer Privacy Act (CCPA), you may record your preference to view or remove your personal information by completing the form below.
