Get in Touch
Have questions? Call or chat with our Patient Advocates for answers.
In 2020, the FDA approved the combination of Opdivo (nivolumab) and Yervoy (ipilimumab) for first-line treatment of adults with pleural mesothelioma. Combining mesothelioma drugs, such as chemotherapy and immunotherapy, can improve survival in some patients.
Written by Karen Selby, RN | Medically Reviewed By Dr. Jeffrey Velotta | Edited By Walter Pacheco | Last Update: June 27, 2024
Many different types of anti-cancer drugs treat mesothelioma with varying degrees of success. Traditional chemotherapy remains the most effective option. Ongoing clinical trials investigate other drugs to find more effective medications for mesothelioma.
Types of Anti-Cancer Mesothelioma Drug Treatments
Any anti-cancer drug is technically considered a form of chemotherapy. The difference between chemotherapy and other anti-cancer drugs is that chemotherapy kills cancer cells, while others block cancer cells from growing or spreading.
Patients can try drugs not approved by the U.S. Food and Drug Administration. They may do so through clinical trials and compassionate-use programs.
Doctors may use these medications in combination with treatments such as surgery and radiation therapy. This combination approach is called multimodal therapy. It attacks the cancer in multiple ways to maximize the chance of killing cancer cells and shrinking tumors.
Chemotherapy is one of the most common treatments for any cancer, including mesothelioma. Despite its well-known side effects, chemotherapy is one of the best mesothelioma treatment options. These drugs seek out and attack rapidly dividing cells, including cancer cells.
Common Chemotherapy Medications for Mesothelioma
A combination of cisplatin and pemetrexed is most effective for most mesothelioma patients. This combination is the most common first-line chemotherapy for people with mesothelioma. Second-line chemotherapy may be an option if the drugs aren’t tolerated or highly effective. Second-line chemotherapy drugs include carboplatin, gemcitabine, vinorelbine (Navelbine) or doxorubicin.
Most chemotherapy drugs are synthetic, but Navelbine is semi-synthetic. Part of the drug comes from a flowering plant known as periwinkle. Patients get Navelbine alone or in combination with another chemotherapy drug, such as cisplatin.
These are potent drugs that may disrupt digestion. Some patients report stomach pain, nausea and irregular stools. Medications are available to control these side effects.
Typical Side Effects of Mesothelioma Chemotherapy Drugs
Patients who develop specific side effects should immediately contact their physician. These include fever, chills, rash, swollen ankles and blood in their urine.
The kidneys clear chemotherapy medications from your blood and prevent toxicity. The chemotherapy drugs pemetrexed, cisplatin and carboplatin are potentially harmful to your kidneys. This risk intensifies if you take certain medications at the same time, including:
This list is not all-inclusive. Many medications can cause kidney damage in combination with chemotherapy for mesothelioma. The safest thing to do is ask the doctor who prescribed the chemotherapy what medications will be safe to take during treatment.
Alert your doctor immediately if you experience symptoms of kidney damage. Signs include little or no urination, abdominal swelling or rapid weight gain.
Other types of drugs can be dangerous for mesothelioma patients receiving chemotherapy. Vitamin B6 supplements and certain seizure medications can interact negatively with cisplatin.
Chemotherapy patients are strongly advised not to receive live vaccines. Examples include the shingles vaccine or the nasal flu vaccine. The injectable flu shots contain inactivated virus particles. Your doctor may advise you to have that injection yearly.

Get specialized treatment from experienced mesothelioma doctors.
Find a Doctor NowImmunotherapy agents enhance a patient’s immune system and help the body attack cancer cells. The immune system often does not recognize cancer cells as unhealthy cells. However, with the addition of immunotherapy medications, the immune system may begin fighting the cancer.
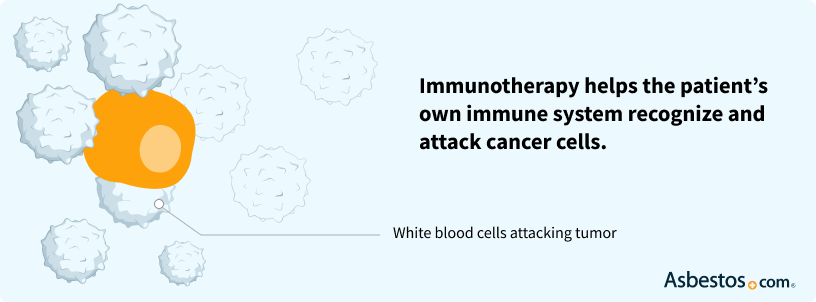
Interferons and interleukin-12 also have been studied for the treatment of malignant mesothelioma.
CRS-207 is an immunotherapy vaccine derived from a bacterium. It is in a phase I mesothelioma trial. Preliminary results became available in 2016, showing that 94% of participants responded to the drug. Tumor shrinkage occurred for 85% of participants, and 35% saw no new tumor growth. Clinical trials of CRS-207 in mesothelioma are ongoing and promising.
Amatuximab (MORAb-009) is an immunotherapy drug tested on mesothelioma patients. Despite promising results in the phase I trial, the phase II trial failed to warrant further study.
I guess I’m an anomaly. I’m doing great right now. I really am. I don’t look like a stage 4 cancer patient.Andy A.Pleural mesothelioma survivor and amatuximab patient diagnosed in 2010
The overall median survival was 14.8 months. However, a 2021 study of Amatuximab showed that it successfully inhibited cancer cells in the peritoneum.
To date, an ongoing phase I study of SS1P in people with pleural mesothelioma has reported a partial tumor response in 12 out of 20 participants.
Immunotherapy drugs are appealing because they have milder side effects than chemotherapy.
Targeted therapy is a growing field in cancer treatment that targets specific molecular changes involved in cancer cell development, growth, metastasis and death. While chemotherapy affects healthy and cancerous cells, targeted therapy is designed only to affect cancerous cells.
NGR-hTNF is a targeted therapy drug currently in phase III trials to determine its efficacy as a management therapy for people with recurring mesothelioma. In the phase II trial, overall survival was 12 months among patients formerly treated with pemetrexed chemotherapy. The phase III trial is ongoing, and current data shows an overall survival of 11.7 months among certain patients. This preliminary data offers hope for more effective second-line therapy for recurring mesothelioma that will buy patients more time.
This targeted drug blocks heat shock protein 90, which promotes mesothelioma cell growth. A phase I and II clinical trial of ganetespib in pleural mesothelioma patients is ongoing in the U.K. The trial is testing ganetespib as an ongoing maintenance therapy after combination treatment with ganetespib and chemotherapy.
This drug targets EZH2, an enzyme that helps mesothelioma cancer cells grow and divide. The drug seems to work best in patients with the BAP-1 gene mutation, which is present in 60% of mesothelioma cases. Two clinical trials are investigating tazemetostat in mesothelioma patients. The first accepts participants with or without the BAP-1 mutation, while the second only includes mesothelioma patients with a BAP-1 mutation.
Another form of targeted therapy tested in clinical trials on mesothelioma patients is ranpirnase (Onconase). The drug is among the few therapies progressing to a phase III clinical trial. Though early results were promising, preliminary phase IIIb clinical trial results showed that Onconase did not improve overall survival enough for the FDA to award approval.
Following the Onconase trials, mesothelioma researchers — among them Dr. Michele Carbone and Dr. Harvey Pass — performed a study on mesothelioma cell lines in 2011 to see whether Onconase could be more effective in mesothelioma patients with low levels of the protein kinase enzyme Akt. Results from the study suggest that Onconase could provide slight therapeutic benefit to that group, though no further studies have occurred to investigate this potential in humans.
Another study tested the combination of Onconase and an anti-malarial drug in test tubes and mice with mesothelioma. This 2016 Chinese study showed a significant decrease in tumors and an anti-angiogenesis effect. Further research is necessary to test this effect in humans.

Targeted drugs are great for mesothelioma patients. They specifically target the cancer cells and don’t affect normal tissue, which is significant. The benefit is that they reduce the adverse effects on healthy tissue, increase treatment effectiveness, improve outcomes and lead to a better quality of life for the patient.
Scientists continue searching for effective drugs to help prevent angiogenesis, a function that allows the body to heal by creating new blood vessels. The process also builds vessels to tumors, feeding them with a fresh blood supply and allowing them to grow and spread.
Researchers believe that stopping this process may be the key to stopping the aggressive nature of mesothelioma and the spread of many other types of cancer. Angiogenesis is also a necessary precursor to metastasis, the process of cancer cells spreading from one area of the body to another.
Drugs that could inhibit the formation of new blood vessels to tumors are called anti-angiogenesis drugs or angiogenesis inhibitors. An example of an anti-angiogenesis agent is bevacizumab. Without blood vessels to bring nutrients, the cancer cells cannot divide and spread, eventually dying. Stopping angiogenesis is not the hard part. The hard part is stopping angiogenesis related to cancer without hindering the natural ability to heal itself. This challenge is tough and is at the center of considerable research.
Combined with other treatments, such as chemotherapy and radiation, angiogenesis inhibitors are extending life among certain mesothelioma patients in clinical trials. They don’t seem to work consistently in everyone, but some mesothelioma patients have lived years longer because of these drugs. For example, Avastin, thalidomide, sorafenib and cediranib have improved survival rates for certain mesothelioma patients in clinical trials.
Prior studies of Avastin, also known as bevacizumab, in mesothelioma clinical trials produced inconsistent results among participants. Some patients responded well, others not at all. However, a 2015 French study tested the drug on hundreds of mesothelioma patients in combination with chemotherapy and reported an overall survival of 18.8 months. Average survival is around 12 months with chemotherapy alone.
Access top mesothelioma cancer centers that have experience treating this rare disease.
Get Help NowAngiogenesis occurs using a complex “s” signaling cascade” in which cells communicate messages via proteins. Anti-angiogenesis drugs interfere with this process in one of three ways, each corresponding to a specific step of the cascade. This class of drugs is technically considered a form of targeted therapy.
The first step of the angiogenesis process makes room for new endothelial cells located on the outer lining of blood vessels. In this step, endothelial cells break down placeholder material surrounding the cells, called the extracellular matrix.
Cancer cells trigger this process to create space for a new blood vessel to branch off an existing one toward a tumor. When anti-angiogenesis drugs inhibit this process, new cells have no room to form blood vessels, preventing the angiogenesis cascade.
Anti-angiogenesis drugs tend to cause fewer and milder side effects than chemotherapy. Whereas chemotherapy drugs attack quickly dividing cells, even if they are healthy, anti-angiogenesis drugs do not affect most healthy cells.
The downside is that because angiogenesis occurs when wounds are healing, these drugs complicate the healing process and may lead to excessive bleeding. Patients may also develop blood clots in the arteries, internal bleeding or a hole in the digestive tract.
Overall, angiogenesis-targeting therapies have a low risk of significant side effects while showing promising results in preventing mesothelioma metastasis.
Photodynamic therapy is a two-step treatment procedure that uses photosensitizing drugs to make cancer cells vulnerable to light and then uses light to destroy the cancer cells. For mesothelioma patients, the photosensitizer is almost always porfimer sodium (Photofrin), which is also used in other cancers, like esophageal cancer.
Researchers are testing photodynamic therapy in pleural mesothelioma patients and have discovered that it improves survival times. The treatment is still in development for peritoneal mesothelioma while doctors search for an effective means of administering light to the abdominal cavity. The therapy commonly causes light sensitivity for about six weeks, possibly resulting in burning, swelling or scarring.
The landscape of new mesothelioma treatments is constantly evolving.
Oncologists say the future of all cancer treatment is personalization. The one-size-fits-all approach is a thing of the past. In the future, doctors will examine your genes, the DNA in your tumors and biomarkers in your blood to decipher which drugs will directly target your cancer.
Before starting any medication, discuss it thoroughly with your mesothelioma oncologist. Ask these questions:
I can’t stress enough how important it is to do your own research. Keep a journal or notebook of drugs and treatments your doctor mentions to look into more when you get home. You must weigh the pros and cons of each option.
More participants are needed for mesothelioma clinical trials to get the research we need. Consider speaking with your oncologist about which trials are right for you.
Recommended ReadingThank you for your feedback. Would you like to speak with a Patient Advocate?
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, June 27). Mesothelioma Medications. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/drugs/
Selby, Karen. "Mesothelioma Medications." Asbestos.com, 27 Jun 2024, https://www.asbestos.com/treatment/drugs/.
Selby, Karen. "Mesothelioma Medications." Asbestos.com. Last modified June 27, 2024. https://www.asbestos.com/treatment/drugs/.
A medical doctor who specializes in mesothelioma or cancer treatment reviewed the content on this page to ensure it meets current medical standards and accuracy.
Please read our editorial guidelines to learn more about our content creation and review process.
Dr. Jeffrey Velotta is an experienced thoracic surgeon and pleural mesothelioma specialist at Kaiser Permanente Oakland Medical Center in California. Velotta also serves as an assistant professor at the University of California, San Francisco.
A medical doctor who specializes in mesothelioma or cancer treatment reviewed the content on this page to ensure it meets current medical standards and accuracy.
Please read our editorial guidelines to learn more about our content creation and review process.
Dr. Jeffrey Velotta is an experienced thoracic surgeon and pleural mesothelioma specialist at Kaiser Permanente Oakland Medical Center in California. Velotta also serves as an assistant professor at the University of California, San Francisco.
Mesothelioma Center - Vital Services for Cancer Patients & Families doesn’t believe in selling customer information. However, as required by the new California Consumer Privacy Act (CCPA), you may record your preference to view or remove your personal information by completing the form below.
