CTLA-4 and Mesothelioma
CTLA-4 is a protein that plays an important role in the immune system. It helps keep the immune system in check by regulating T cells. Ipilimumab (Yervoy) and tremelimumab are two immune checkpoint inhibitor drugs that block CTLA-4 to allow T cells to find and fight cancers such as mesothelioma.
What Is CTLA-4?
Cytotoxic T-lymphocyte-associated protein 4, or CTLA-4, is a protein on the surface of T cells that functions as an immune checkpoint. It affects how T cells recognize cancer cells.
T cells attack foreign invaders, such as viruses and cancer cells.
CTLA-4 prevents T cells from attacking healthy parts of the body, but it can also prevent T cells from identifying and killing cancer cells.
Drugs that block CTLA-4 are called immune checkpoint inhibitors. These drugs have the potential to help people live longer with mesothelioma cancer.
Targeting CTLA-4 with immune checkpoint inhibitors is a form of immunotherapy. New immunotherapies are among the most promising mesothelioma treatments in development right now.

Connect with trusted specialists who truly care about your health. Get fast, stress-free appointment help.
Find a Doctor NowHow CTLA-4 Works?
French researchers discovered CTLA-4 in 1987. Nearly a decade later, scientists at the University of California, Berkley, realized the role of CTLA-4.
The protein acts as a break, stopping T cells from attacking cancer cells. Researchers call this breaking mechanism an immune checkpoint.
Immune checkpoints work like on and off switches, activating or deactivating the parts of the immune system.
When activated, CTLA-4 downregulates the immune system by stopping T cells from finding cancer cells. In 2021, a clinical research study concluded that patients who express higher levels of CTLA-4 may have decreased mesothelioma survival times.
Scientists have developed drugs to block and deactivate CTLA-4 — a process commonly called a CTLA-4 blockade — which allows T cells to find and attack cancer cells. These drugs are known as immune checkpoint inhibitors.
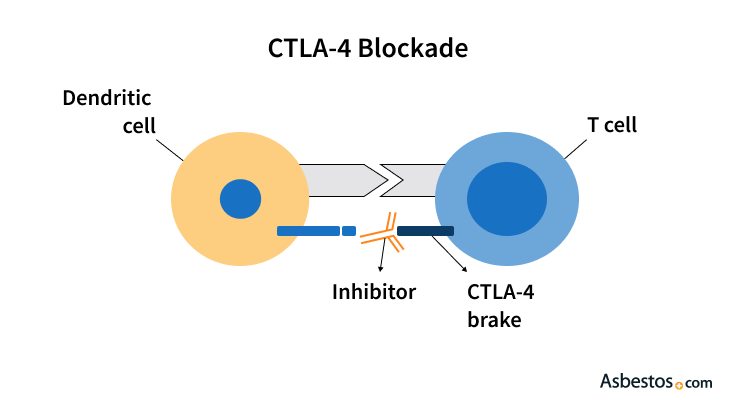
Other immune checkpoint inhibitors targeted in mesothelioma treatment include PD-1 and PD-L1. Drugs that target these checkpoints include Keytruda (Pembrolizumab), Opdivo (Nivolumab) and Imfinzi (Durvalumab).
What Drugs Target CTLA-4?
Two checkpoint inhibitor drugs work against CTLA-4: Yervoy and tremelimumab.
Yervoy is FDA-approved to treat late-stage melanoma. Tremelimumab is not FDA-approved, but in 2015 it received orphan drug designation to encourage research into it.
Anti-CTLA-4 Immunotherapy for Mesothelioma
Most of the mesothelioma clinical trials involving Yervoy and tremelimumab combine them with immune checkpoint inhibitors that target other proteins. Studies show that targeting PD-1 and CTLA-4 together increases effectiveness.
Mesothelioma researchers use the combination approach because it has proven more effective in melanoma treatment. For example, when used alone, about 11 percent of melanoma patients respond to Yervoy.
When the anti-PD-1 drug Opdivo is added to Yervoy, about 61% of melanoma patients respond.
Researchers found that adding Opdivo to Yervoy increases the response rate in mesothelioma patients. 2017 French researchers announced a significant improvement in response rates at the 2017 ASCO Annual Meeting.
Approximately 44% of mesothelioma patients had control of their cancer with Opdivo alone, while 50% had control of their cancer using Yervoy combined with Opdivo.
These results justified a phase III trial of Yervoy and Opdivo in mesothelioma patients, which began in 2017.
Less research is available on tremelimumab than Yervoy, but preliminary results show further research is warranted.
Clinical trials are investigating tremelimumab among mesothelioma patients in the U.S.
- One involves combining tremelimumab with surgery.
- Another is combining tremelimumab with durvalumab (Imfinzi).
Clinical trials suggest that Yervoy and tremelimumab are effective for some people with mesothelioma. Further research is necessary to determine which mesothelioma patients may benefit the most from immune checkpoint inhibitors that target CTLA-4.
Additional research is also required to see which drug combinations work best among people with mesothelioma.
Recommended Reading


