Get in Touch
Have questions? Call or chat with our Patient Advocates for answers.
Virotherapy is a cancer treatment that uses oncolytic viruses and viral immunotherapy to destroy cancer cells directly or trigger the immune system to find and kill cancer cells. Clinical trials are studying virotherapy targeting mesothelioma cancer.

Written by Karen Selby, RN | Medically Reviewed By Dr. Andrea Wolf | Edited By Walter Pacheco | Last Update: July 2, 2024
Virotherapy is a cancer treatment that uses a virus to find and destroy cancer cells. It’s a targeted treatment that can deliver therapy without harming healthy cells.
Types of virotherapy include oncolytic virotherapy and viral immunotherapy. Another type is viral vectors, which is also called viral gene therapy.
Viral vectors also can deliver tumor antigens into the body. Antigens are substances found on cancer cells but not on normal cells. This stimulates an antitumor immune response.
This is similar to how viral immunotherapy works. Sometimes the terms “viral vector therapy” and “viral immunotherapy” are used interchangeably.
Virotherapy is not a cure for mesothelioma. Researchers hope combining it with other therapies will help cancer patients live longer.
More than a century ago, a surgeon named William Coley developed virotherapy. He noticed that cancer patients with an infection after surgery sometimes lived longer than those without.
Dr. Coley wondered if the infection triggered the patients’ immune system to attack the cancer. He developed experimental treatments called Coley’s toxins. Some consider these to be one of the first immunotherapy treatments.
Coley’s toxins first used live bacteria injected into tumors. Later, he used heat-killed microbes. The results were unpredictable. Some patients experienced severe infections and died after Coley’s treatments. Over time, the approach fell from favor.
Fortunately, Coley’s early work was not wholly forgotten. It laid the foundation for developing today’s immunotherapy and virotherapy cancer treatments.
Access top mesothelioma cancer centers that have experience treating this rare disease.
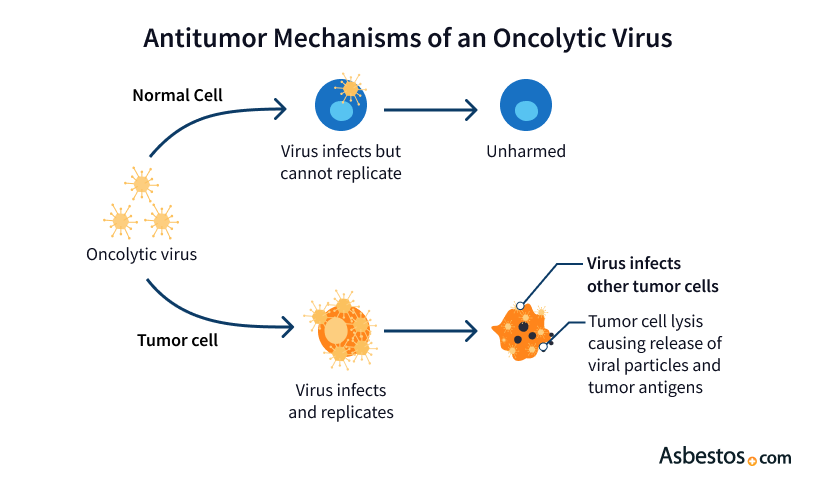
Get Help NowOncolytic viruses infect cancer cells. Some viruses naturally infect cancer cells. Other viruses can be modified in the lab to target specific cancer cell types.
The viruses make their way into cancer cells and reproduce rapidly. The rapid viral reproduction ruptures the membranes of cancer cells and destroys them.
The destroyed cancer cells release antigens. These are substances more easily recognized as foreign by the body. This stimulates the immune system to attack remaining tumors, too.
Oncolytic viruses seem to destroy tumors in two ways. Firstly, by directly rupturing cancer cell membranes. Secondly, they indirectly stimulate the immune system to recognize and attack the cancer.
Researchers are also developing methods to combine gene therapy and virotherapy using oncolytic viruses. One example includes modifying oncolytic viruses to deliver p53 genes. These genes help the immune system fight cancer.
Viral immunotherapy uses a virus to deliver an immune-stimulating substance called an antigen into the body. The antigen helps the immune system recognize and attack cancer cells.
A 2014 phase I clinical trial report published in the medical journal Oncoimmunology describes this type of virotherapy. Researchers evaluated the case of a 68-year-old man with asbestos-related malignant pleural mesothelioma (MPM).
The clinical trial used a type of virus called an adenovirus. The treatment is called ONCOS-102.
When a response is better than expected, the doctors may publish a report detailing these results. In the ONCOS-102 clinical trial, the man survived 18 months from treatment beginning and more than 33 months from diagnosis.
The study scientists reported the response as “remarkable,” given the median survival of patients with MPM varies from 4 to 12 months from diagnosis.
Phase I studies test the safety, side effects, best dose and timing of a new treatment. The dose may be increased slowly to find the highest amount that does not cause harmful side effects.
Although phase I clinical trials are not designed to treat or cure patients, the ONCOS-12 clinical trial seemed to treat one participant more effectively than conventional therapy.
Viral vectors are created in the lab. Researchers start with a typical virus and alter it to create one that cannot cause disease.
This type of virotherapy is considered a form of gene therapy because the modified viruses alter genes in targeted cells. Targets can include cancer cells or other malfunctioning cells contributing to genetic diseases.
Some viral vectors target malfunctioning mesothelioma genes. One example of this approach uses a virus to disrupt how mesothelioma cells create the proteins that allow uncontrolled cell growth — a hallmark of all cancers.
In another approach, scientists have created a virus targeting mesothelioma genes that make too much of one growth factor. The virus only infects the cells with excessive amounts of the growth-promoting substance.
Viral vectors can kill cancer cells directly or make them more sensitive to conventional treatments such as chemotherapy and radiation therapy.
Promising viruses for mesothelioma virotherapy include modified versions of vaccinia, measles, adenovirus, herpes simplex and Newcastle disease virus.
These approaches have been studied in clinical trials, though none is approved for widespread clinical use yet. These treatments are only available to mesothelioma patients participating in clinical trials.
A 2021 clinical research study studied the CpHV-1 virus as a potential new mesothelioma virotherapy approach. The researchers found that CpHV-1 strongly synergized with cisplatin, a standard chemotherapy medication, and this agent combination did not affect healthy mesothelial cells.
Clinicaltrials.gov currently lists numerous clinical trials using virotherapy approaches. Searching for “mesothelioma and virotherapy” will return results on these trials.
These studies are funded by pharmaceutical companies, cancer centers and government research. For example, the National Cancer Institute has funded some of these studies, including a virotherapy mesothelioma clinical trial.
Additionally, specific research centers are conducting virotherapy research for mesothelioma. For example, the National Institutes of Health and Vaccine Immunotherapy Center conduct mesothelioma research on immunotherapy and virotherapy.
There are few studies on combinations of virotherapy and chemotherapy. Preclinical (animal) research suggests this approach may be very effective for treating mesothelioma.
One example of preclinical combination therapy research studied mesothelioma virotherapy plus pemetrexed (Alimta) and cisplatin or carboplatin. Pemetrexed and platin chemotherapy drugs are the standards of care.
Medical experts accept this standard of care as appropriate treatment for particular cancer. It is widely used by medical oncology professionals and typically is the first treatment offered when a person is diagnosed with mesothelioma.
This study found that chemotherapy alone did not decrease mesothelioma tumor growth. However, the virotherapy alone slowed tumor growth, and the combination of virotherapy plus chemotherapy delayed tumor growth even more.
The safety and immune-activating properties of this virotherapy in humans are already known. Chemotherapy is already the standard of care and is widely used to treat mesothelioma.
These factors provide a strong rationale for clinical trials testing virotherapy with first-line chemotherapy in patients with malignant mesothelioma. These trials likely will be coming soon.
Recommended ReadingThank you for your feedback. Would you like to speak with a Patient Advocate?
Your web browser is no longer supported by Microsoft. Update your browser for more security, speed and compatibility.
If you are looking for mesothelioma support, please contact our Patient Advocates at (855) 404-4592
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
The Mesothelioma Center at Asbestos.com has provided patients and their loved ones the most updated and reliable information on mesothelioma and asbestos exposure since 2006.
Our team of Patient Advocates includes a medical doctor, a registered nurse, health services administrators, veterans, VA-accredited Claims Agents, an oncology patient navigator and hospice care expert. Their combined expertise means we help any mesothelioma patient or loved one through every step of their cancer journey.
More than 30 contributors, including mesothelioma doctors, survivors, health care professionals and other experts, have peer-reviewed our website and written unique research-driven articles to ensure you get the highest-quality medical and health information.
My family has only the highest compliment for the assistance and support that we received from The Mesothelioma Center. This is a staff of compassionate and knowledgeable individuals who respect what your family is experiencing and who go the extra mile to make an unfortunate diagnosis less stressful. Information and assistance were provided by The Mesothelioma Center at no cost to our family.LashawnMesothelioma patient’s daughter


Selby, K. (2024, July 2). Virotherapy Treatment for Mesothelioma Patients. Asbestos.com. Retrieved July 26, 2024, from https://www.asbestos.com/treatment/virotherapy/
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com, 2 Jul 2024, https://www.asbestos.com/treatment/virotherapy/.
Selby, Karen. "Virotherapy Treatment for Mesothelioma Patients." Asbestos.com. Last modified July 2, 2024. https://www.asbestos.com/treatment/virotherapy/.
A medical doctor who specializes in mesothelioma or cancer treatment reviewed the content on this page to ensure it meets current medical standards and accuracy.
Please read our editorial guidelines to learn more about our content creation and review process.
Dr. Andrea Wolf is the Director of the New York Mesothelioma Program at Mount Sinai in New York City. She focuses on multidisciplinary treatment, clinical research, community outreach and education.
A medical doctor who specializes in mesothelioma or cancer treatment reviewed the content on this page to ensure it meets current medical standards and accuracy.
Please read our editorial guidelines to learn more about our content creation and review process.
Dr. Andrea Wolf is the Director of the New York Mesothelioma Program at Mount Sinai in New York City. She focuses on multidisciplinary treatment, clinical research, community outreach and education.
Mesothelioma Center - Vital Services for Cancer Patients & Families doesn’t believe in selling customer information. However, as required by the new California Consumer Privacy Act (CCPA), you may record your preference to view or remove your personal information by completing the form below.
